Glycopolymers for Antibacterial and Antiviral Applications
- PMID: 36770653
- PMCID: PMC9919862
- DOI: 10.3390/molecules28030985
Glycopolymers for Antibacterial and Antiviral Applications
Abstract
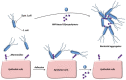
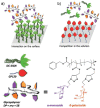
Diseases induced by bacterial and viral infections are common occurrences in our daily life, and the main prevention and treatment strategies are vaccination and taking antibacterial/antiviral drugs. However, vaccines can only be used for specific viral infections, and the abuse of antibacterial/antiviral drugs will create multi-drug-resistant bacteria and viruses. Therefore, it is necessary to develop more targeted prevention and treatment methods against bacteria and viruses. Proteins on the surface of bacteria and viruses can specifically bind to sugar, so glycopolymers can be used as potential antibacterial and antiviral drugs. In this review, the research of glycopolymers for bacterial/viral detection/inhibition and antibacterial/antiviral applications in recent years are summarized.
Keywords: antibacterial; antivirus; detection; glycopolymer; inhibition; lectin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















References
-
- Behren S., Westerlind U. Novel approaches to design glycan-based antibacterial inhibitors. Eur. J. Org. Chem. 2022;26:e202200795. doi: 10.1002/ejoc.202200795. - DOI
-
- Kiessling L.L., Pontrello J.K., Schuster M.C. Synthetic multivalent carbohydrate ligands as effectors or inhibitors of biological processes. In: Wong C.-H., editor. Carbohydrate-Based Drug Discovery. Wiley; Hoboken, NJ, USA: 2003. pp. 575–608.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

