The Pharmaceutical Industry in 2022: An Analysis of FDA Drug Approvals from the Perspective of Molecules
- PMID: 36770706
- PMCID: PMC9921400
- DOI: 10.3390/molecules28031038
The Pharmaceutical Industry in 2022: An Analysis of FDA Drug Approvals from the Perspective of Molecules
Abstract
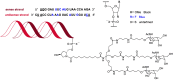
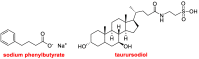
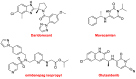
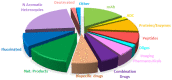
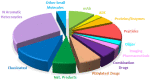
While 2021 ended with the world engulfed in the COVID-19 Omicron wave, 2022 has ended in almost all countries, except China, with COVID-19 being likened to the flu. In this context, the U.S. Food and Drug Administration (FDA) has authorized only 37 new drugs this year compared to an average of 52 in the last four years. Thus 2022 is the second lowest harvest after 2016 in the last six years. This ranking may be transient and will be confirmed in the coming years. In this regard, the reduction in the number of drugs accepted by the FDA this year applies only to the so-called small molecules as there has been no variation in the respective numbers of biologics or TIDES (peptides and oligonucleotides). Monoclonal antibodies (mAbs) continue to be the class with the most drugs authorized (9), while proteins/enzymes (5) and an antibody-drug conjugate complete the biologics harvest. In 2022, five TIDES and seven drugs inspired by natural products have received the green light, thus showing the same tendency as in previous years. Finally, pharmaceutical agents with nitrogen aromatic heterocycles and/or fluorine atoms continue to be predominant among small molecules this year. Furthermore, three drugs have been approved for imaging, reinforcing the trend in recent years for this class of treatments. A keyword in 2022 is bispecificity since four drugs have this property (two mAbs, one protein, and one peptide). Herein, the 37 new drugs approved by the FDA in 2022 are analyzed. On the basis of chemical structure alone, these drugs are classified as the following: biologics (antibodies, antibody-drug conjugates, proteins/enzymes), TIDES (peptide and oligonucleotides), combined drugs, natural products; nitrogen aromatic heterocycles, fluorine-containing molecules, and other small molecules.
Keywords: TIDES; antibodies; biologics; chemical entities; fluorine-based drugs; imaging; natural products; new chemical entities; oligonucleotides; peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



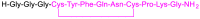






References
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2023)]; Available online: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-f....
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2023)]; Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and....
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2023)]; Available online: https://www.fda.gov/vaccines-blood-biologics/development-approval-proces....
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2023)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/counterterrorism....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

