Antioxidant, Wound Healing Potential and In Silico Assessment of Naringin, Eicosane and Octacosane
- PMID: 36770709
- PMCID: PMC9919607
- DOI: 10.3390/molecules28031043
Antioxidant, Wound Healing Potential and In Silico Assessment of Naringin, Eicosane and Octacosane
Abstract
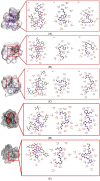
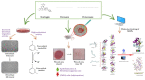
1. Diabetic chronic wounds, mainly foot ulcers, constitute one of the most common complications of poorly managed diabetes mellitus. The most typical reasons are insufficient glycemic management, latent neuropathy, peripheral vascular disease, and neglected foot care. In addition, it is a common cause of foot osteomyelitis and amputation of the lower extremities. Patients are admitted in larger numbers attributable to chronic wounds compared to any other diabetic disease. In the United States, diabetes is currently the most common cause of non-traumatic amputations. Approximately five percent of diabetics develop foot ulcers, and one percent require amputation. Therefore, it is necessary to identify sources of lead with wound-healing properties. Redox imbalance due to excessive oxidative stress is one of the causes for the development of diabetic wounds. Antioxidants have been shown to decrease the progression of diabetic neuropathy by scavenging ROS, regenerating endogenous and exogenous antioxidants, and reversing redox imbalance. Matrix metalloproteinases (MMPs) play vital roles in numerous phases of the wound healing process. Antioxidant and fibroblast cell migration activity of Marantodes pumilum (MP) crude extract has previously been reported. Through their antioxidant, epithelialization, collagen synthesis, and fibroblast migration activities, the authors hypothesise that naringin, eicosane and octacosane identified in the MP extract may have wound-healing properties. 2. The present study aims to identify the bioactive components present in the dichloromethane (DCM) extract of M. pumilum and evaluate their antioxidant and wound healing activity. Bioactive components were identified using LCMS, HPTLC and GCMS. Excision wound on STZ-induced diabetic rat model, human dermal fibroblast (HDF) cell line and colorimetric antioxidant assays were used to evaluate wound healing and antioxidant activities, respectively. Molecular docking and pkCMS software would be utilised to predict binding energy and affinity, as well as ADME parameters. 3. Naringin (NAR), eicosane (EIC), and octacosane (OCT) present in MP displayed antioxidant action and wound excision closure. Histological examination HDF cell line demonstrates epithelialization, collagen production, fibroblast migration, polymorphonuclear leukocyte migration (PNML), and fibroblast movement. The results of molecular docking indicate a substantial attraction and contact between MMPs. pkCMS prediction indicates inadequate blood-brain barrier permeability, low toxicity, and absence of hepatotoxicity. 4. Wound healing properties of (NEO) naringin, eicosane and octacosane may be the result of their antioxidant properties and possible interactions with MMP.
Keywords: MMPs; antioxidants; diabetes mellitus; eicosane; naringin; octacosane; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Oliver T.I., Mutluoglu M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. [(accessed on 10 December 2022)]. Diabetic Foot Ulcer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537328/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

