Chemical Modification of Auranofin Yields a New Family of Anticancer Drug Candidates: The Gold(I) Phosphite Analogues
- PMID: 36770719
- PMCID: PMC9920260
- DOI: 10.3390/molecules28031050
Chemical Modification of Auranofin Yields a New Family of Anticancer Drug Candidates: The Gold(I) Phosphite Analogues
Abstract
A panel of four novel gold(I) complexes, inspired by the clinically established gold drug auranofin (1-Thio-β-D-glucopyranosatotriethylphosphine gold-2,3,4,6-tetraacetate), was prepared and characterized. All these compounds feature the replacement of the triethylphosphine ligand of the parent compound auranofin with a trimethylphosphite ligand. The linear coordination around the gold(I) center is completed by Cl-, Br-, I- or by the thioglucose tetraacetate ligand (SAtg). The in-solution behavior of these gold compounds as well as their interactions with some representative model proteins were comparatively analyzed through 31PNMR and ESI-MS measurements. Notably, all panel compounds turned out to be stable in aqueous media, but significant differences with respect to auranofin were disclosed in their interactions with a few leading proteins. In addition, the cytotoxic effects produced by the panel compounds toward A2780, A2780R and SKOV-3 ovarian cancer cells were quantitated and found to be in the low micromolar range, since the IC50 of all compounds was found to be between 1 μM and 10 μM. Notably, these novel gold complexes showed large and similar inhibition capabilities towards the key enzyme thioredoxin reductase, again comparable to those of auranofin. The implications of these results for the discovery of new and effective gold-based anticancer agents are discussed.
Keywords: anticancer compounds; auranofin; metal-based drugs; phosphite compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



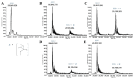
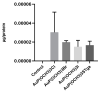

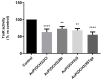
References
-
- [(accessed on 22 December 2022)]; Available online: https://www.clinicaltrials.gov/ct2/results?cond=&term=auranofin&cntry=&s...
-
- Hill D.T., Isab A.A., Griswold D.E., DiMartino M.J., Matz E.D., Figueroa A.L., Wawro J.E., DeBrosse C., Reiff W.M., Elder R.C., et al. Seleno-Auranofin (Et3PAuSe-tagl): Synthesis, Spectroscopic (EXAFS, 197Au Mössbauer, 31P, 1H, 13C, and 77Se NMR, ESI-MS) Characterization, Biological Activity, and Rapid Serum Albumin-Induced Triethylphosphine Oxide Generation. Inorg. Chem. 2010;49:7663–7675. doi: 10.1021/ic902335z. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

