Efficient Optosensing of Hippuric Acid in the Undiluted Human Urine with Hydrophilic "Turn-On"-Type Fluorescent Hollow Molecularly Imprinted Polymer Microparticles
- PMID: 36770744
- PMCID: PMC9920520
- DOI: 10.3390/molecules28031077
Efficient Optosensing of Hippuric Acid in the Undiluted Human Urine with Hydrophilic "Turn-On"-Type Fluorescent Hollow Molecularly Imprinted Polymer Microparticles
Abstract
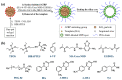
The development of complex biological sample-compatible fluorescent molecularly imprinted polymers (MIPs) with improved performances is highly important for their real-world bioanalytical and biomedical applications. Herein, we report on the first hydrophilic "turn-on"-type fluorescent hollow MIP microparticles capable of directly, highly selectively, and rapidly optosensing hippuric acid (HA) in the undiluted human urine samples. These fluorescent hollow MIP microparticles were readily obtained through first the synthesis of core-shell-corona-structured nitrobenzoxadiazole (NBD)-labeled hydrophilic fluorescent MIP microspheres by performing one-pot surface-initiated atom transfer radical polymerization on the preformed "living" silica particles and subsequent removal of their silica core via hydrofluoric acid etching. They showed "turn-on" fluorescence and high optosensing selectivity and sensitivity toward HA in the artificial urine (the limit of detection = 0.097 μM) as well as outstanding photostability and reusability. Particularly, they exhibited much more stable aqueous dispersion ability, significantly faster optosensing kinetics, and higher optosensing sensitivity than their solid counterparts. They were also directly used for quantifying HA in the undiluted human urine with good recoveries (96.0%-102.0%) and high accuracy (RSD ≤ 4.0%), even in the presence of several analogues of HA. Such fluorescent hollow MIP microparticles hold much promise for rapid and accurate HA detection in the clinical diagnostic field.
Keywords: complex biological samples; fluorescence “turn-on”; hippuric acid; hollow; human urine sample; molecularly imprinted polymers; sacrificial template method; surface-initiated ATRP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hoshino Y., Shea K.J. The evolution of plastic antibodies. J. Mater. Chem. 2011;21:3517–3521. doi: 10.1039/C0JM03122D. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

