Tuning Photochemical and Photophysical Properties of P(V) Phthalocyanines
- PMID: 36770759
- PMCID: PMC9920145
- DOI: 10.3390/molecules28031094
Tuning Photochemical and Photophysical Properties of P(V) Phthalocyanines
Abstract
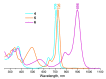
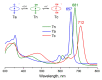
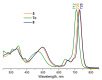
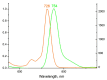
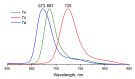
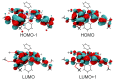
The ability of P(V) phthalocyanines (Pcs) for efficient singlet oxygen (SO) generation was demonstrated for the first time by the example of unsubstituted and α- and β-octabutoxy-substituted P(V)Pcs with hydroxy, methoxy and phenoxy ligands in the apical positions of the octahedral P centre. Variation of substituents in Pc ring and P(V) axial ligands allows careful tuning of photophysical and photochemical properties. Indeed, a combination of BuO groups in the β-positions of the Pc ring and PhO groups as axial ligands provides significant SO generation quantum yields up to 90%; meanwhile, the values of SO generation quantum yields for others investigated compounds vary from 27 to 55%. All the complexes, except α-substituted P(V)Pc, demonstrate fluorescence with moderate quantum yields (10-16%). The introduction of electron-donating butoxy groups, especially in the α-position, increases the photostability of P(V)Pcs. Moreover, it has been shown in the example of β-BuO-substituted P(V) that the photostability depends on the nature of axial ligands and increases in the next row: OPh < OMe < OH. The presence of oxy/hydroxy axial ligands on the P(V) atom makes it possible to switch the photochemical and photophysical properties of P(V)Pcs by changing the acidity of the media.
Keywords: fluorescence; phosphorus; photostability; phthalocyanine; singlet oxygen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fukuda T., Kobayashi N. Handbook of Porphyrin Science. World Scientific Publishing; Singapore: 2010. UV-Visible Absorption Spectroscopic Properties of Phthalocyanines and Related Macrocycles; pp. 1–644.
-
- Nyokong T. Effects of Substituents on the Photochemical and Photophysical Properties of Main Group Metal Phthalocyanines. Coord. Chem. Rev. 2007;251:1707–1722. doi: 10.1016/j.ccr.2006.11.011. - DOI
-
- Nyokong T., Antunes E.M. Handbook of Porphyrin Science. World Scientific Publishing; Singapore: 2010. Photochemical and Photophysical Properties of Metallophthalocyanines; pp. 247–357.
-
- Machacek M., Cidlina A., Novakova V., Svec J., Rudolf E., Miletin M., Kučera R., Simunek T., Zimcik P. Far-Red-Absorbing Cationic Phthalocyanine Photosensitizers: Synthesis and Evaluation of the Photodynamic Anticancer Activity and the Mode of Cell Death Induction. J. Med. Chem. 2015;58:1736–1749. doi: 10.1021/jm5014852. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

