Development and Application of Ruthenium(II) and Iridium(III) Based Complexes for Anion Sensing
- PMID: 36770897
- PMCID: PMC9920910
- DOI: 10.3390/molecules28031231
Development and Application of Ruthenium(II) and Iridium(III) Based Complexes for Anion Sensing
Abstract
Improvements in the design of receptors for the detection and quantification of anions are desirable and ongoing in the field of anion chemistry, and remarkable progress has been made in this direction. In this regard, the development of luminescent chemosensors for sensing anions is an imperative and demanding sub-area in supramolecular chemistry. This decade, in particular, witnessed advancements in chemosensors based on ruthenium and iridium complexes for anion sensing by virtue of their modular synthesis and rich chemical and photophysical properties, such as visible excitation wavelength, high quantum efficiency, high luminescence intensity, long lifetimes of phosphorescence, and large Stokes shifts, etc. Thus, this review aims to summarize the recent advances in the development of ruthenium(II) and iridium(III)-based complexes for their application as luminescent chemosensors for anion sensing. In addition, the focus was devoted to designing aspects of polypyridyl complexes of these two transition metals with different recognition motifs, which upon interacting with different inorganic anions, produces desirable quantifiable outputs.
Keywords: anion sensing; luminescent chemosensors; ruthenium(II)/iridium(III) complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





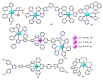
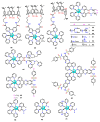
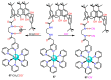
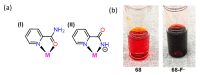

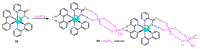
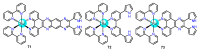
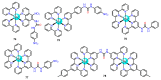



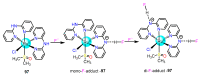

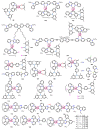
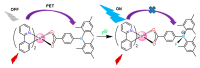
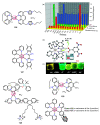

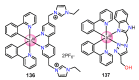

References
-
- Sessler J.L., Gale P.A., Cho W.S. Anion Receptor Chemistry. Royal Society of Chemistry; Cambridge, UK: 2006.
-
- Sessler J.L., Camiolo S., Gale P.A. Pyrrolic and polypyrrolic anion binding agents. Coord. Chem. Rev. 2003;240:17. doi: 10.1016/S0010-8545(03)00023-7. - DOI
-
- Kang S.O., Hossain M.A., Bowman-James K. Influence of dimensionality and charge on anion binding in amide-based macrocyclic receptors. Coord. Chem. Rev. 2006;250:3038–3052. doi: 10.1016/j.ccr.2006.06.006. - DOI
-
- Gale P.A., Quesada R. Anion coordination and anion-templated assembly: Highlights from 2002 to 2004. Coord. Chem. Rev. 2006;250:3219–3244. doi: 10.1016/j.ccr.2006.05.020. - DOI
-
- Gunnlaugsson T., Glynn M., Tocci G.M., Kruger P.E., Pfeffer F.M. Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 2006;250:3094–3117. doi: 10.1016/j.ccr.2006.08.017. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

