In Vitro and In Silico Studies of Neolignans from Magnolia grandiflora L. Seeds against Human Cannabinoids and Opioid Receptors
- PMID: 36770918
- PMCID: PMC9920749
- DOI: 10.3390/molecules28031253
In Vitro and In Silico Studies of Neolignans from Magnolia grandiflora L. Seeds against Human Cannabinoids and Opioid Receptors
Abstract
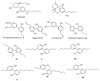
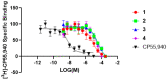
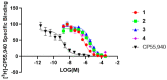
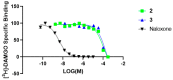
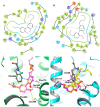
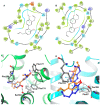
Magnolia grandiflora L. (Magnoliaceae) is a plant of considerable medicinal significance; its flowers and seeds have been used in various traditional remedies. Radioligand binding assays of n-hexane seeds extract showed displacement of radioligand for cannabinoid (CB1 and CB2) and opioid δ (delta), κ (kappa), and µ (mu) receptors. Bioactivity-guided fractionation afforded 4-O-methylhonokiol (1), magnolol (2), and honokiol (3), which showed higher binding to cannabinoid rather than opioid receptors in radioligand binding assays. Compounds 1-3, together with the dihydro analog of 2 (4), displayed selective affinity towards CB2R (Ki values of 0.29, 1.4, 1.94, and 0.99 μM, respectively), compared to CB1R (Ki 3.85, 17.82, 14.55, and 19.08 μM, respectively). An equal mixture of 2 and 3 (1:1 ratio) showed additive displacement activity towards the tested receptors compared to either 2 or 3 alone, which in turn provides an explanation for the strong displacement activity of the n-hexane extract. Due to the unavailability of an NMR or X-ray crystal structure of bound neolignans with the CB1 and CB2 receptors, a docking study was performed to predict ligand-protein interactions at a molecular level and to delineate structure-activity relationships (SAR) of the neolignan analogs with the CB1 and CB2 receptors. The putative binding modes of neolignans 1-3 and previously reported related analogs (4, 4a, 5, 5a, 6, 6a, and 6b) into the active site of the CB1 and CB2 receptors were assessed for the first time via molecular docking and binding free-energy (∆G) calculations. The docking and ∆G results revealed the importance of a hydroxyl moiety in the molecules that forms strong H-bonding with Ser383 and Ser285 within CB1R and CB2R, respectively. The impact of a shift from a hydroxyl to the methoxy group on experimental binding affinity to CB1R versus CB2R was explained through ∆G data and the orientation of the alkyl chain within the CB1R. This comprehensive SAR, influenced by the computational study and the observed in vitro displacement binding affinities, has indicated the potential of magnolia neolignans for developing new CB agonists for potential use as analgesics, anti-inflammatory agents, or anxiolytics.
Keywords: 4-O-methylhonokiol; Magnolia grandiflora; cannabinoid; honokiol; magnolol; molecular docking; opioid; tetrahydromagnolol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

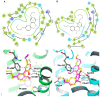
References
-
- Pandey P., Roy K.K., Liu H., Ma G., Pettaway S., Alsharif W.F., Gadepalli R.S., Rimoldi J.M., McCurdy C.R., Cutler S.J., et al. Structure-based identification of potent natural product chemotypes as cannabinoid receptor 1 inverse agonists. Molecules. 2018;23:2630. doi: 10.3390/molecules23102630. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

