Recent Advances in the Synthesis of Cyclic Sulfoximines via C-H Bond Activation
- PMID: 36771034
- PMCID: PMC9921269
- DOI: 10.3390/molecules28031367
Recent Advances in the Synthesis of Cyclic Sulfoximines via C-H Bond Activation
Abstract
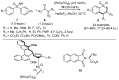
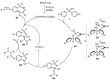
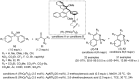
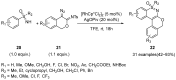
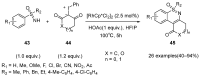
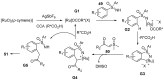
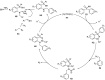
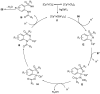
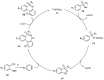
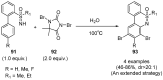
Sulfoximines, a ubiquitous class of structural motifs, are widely present in bioactive molecules and functional materials that have received considerable attention from modern organic chemistry, pharmaceutical industries, and materials science. Sulfoximines have proved to be an effective directing group for C-H functionalization which was widely investigated for the synthesis of cyclic sulfoximines. Within the last decade, great progress has been achieved in the synthesis of cyclic sulfoximines. Thus, this review highlights the recent advances in the synthesis of cyclic sulfoximines via the C-H activation strategy and is classified based on the substrate types.
Keywords: C–H activation; cyclic sulfoximines; cyclization; synthesis; synthetic methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



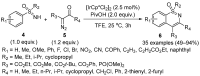

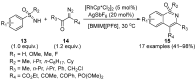













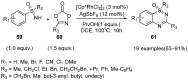








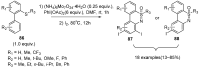


Similar articles
-
Synthesis and Transformations of NH-Sulfoximines.Chemistry. 2021 Dec 9;27(69):17293-17321. doi: 10.1002/chem.202102619. Epub 2021 Oct 13. Chemistry. 2021. PMID: 34519376 Free PMC article. Review.
-
Iridium(III)-Catalyzed C-H Cyclization of Sulfoximines with Diazo Meldrum's Acids for the Synthesis of Cyclic Sulfoximines.J Org Chem. 2023 Aug 18;88(16):11702-11711. doi: 10.1021/acs.joc.3c00984. Epub 2023 Jul 10. J Org Chem. 2023. PMID: 37427877
-
Efficient Synthesis of Cyclic Sulfoximines from N-Propargylsulfinamides through Sulfur-Carbon Bond Formation.Chemistry. 2019 Dec 10;25(69):15755-15758. doi: 10.1002/chem.201904501. Epub 2019 Nov 7. Chemistry. 2019. PMID: 31573124
-
Increasing Complexity: A Practical Synthetic Approach to Three-Dimensional, Cyclic Sulfoximines and First Insights into Their in Vitro Properties.Chemistry. 2020 Apr 1;26(19):4378-4388. doi: 10.1002/chem.201905461. Epub 2020 Mar 9. Chemistry. 2020. PMID: 31961028
-
Advances in cross-coupling and oxidative coupling reactions of NH-sulfoximines - a review.Chem Commun (Camb). 2025 Jan 28;61(10):1934-1943. doi: 10.1039/d4cc05308g. Chem Commun (Camb). 2025. PMID: 39757832 Review.
Cited by
-
Computational Insights into the Formation and Structure of S-N Containing Cyclic Peptides.ACS Omega. 2023 May 11;8(20):18234-18244. doi: 10.1021/acsomega.3c01764. eCollection 2023 May 23. ACS Omega. 2023. PMID: 37251184 Free PMC article.
References
-
- Coquerel Y., Rodriguez J. Chemo- and regioselective synthesis of 2-alkylidenetetrahydrofurans bearing a chiral sulfur atom by domino reactions of sulfoximines. Tetrahedron. Lett. 2008;47:8503–8506. doi: 10.1016/j.tetlet.2006.09.142. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

