The Multifaceted MEP Pathway: Towards New Therapeutic Perspectives
- PMID: 36771066
- PMCID: PMC9919496
- DOI: 10.3390/molecules28031403
The Multifaceted MEP Pathway: Towards New Therapeutic Perspectives
Abstract
Isoprenoids, a diverse class of natural products, are present in all living organisms. Their two universal building blocks are synthesized via two independent pathways: the mevalonate pathway and the 2-C-methyl-ᴅ-erythritol 4-phosphate (MEP) pathway. The presence of the latter in pathogenic bacteria and its absence in humans make all its enzymes suitable targets for the development of novel antibacterial drugs. (E)-4-Hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP), the last intermediate of this pathway, is a natural ligand for the human Vγ9Vδ2 T cells and the most potent natural phosphoantigen known to date. Moreover, 5-hydroxypentane-2,3-dione, a metabolite produced by Escherichia coli 1-deoxy-ᴅ-xylulose 5-phosphate synthase (DXS), the first enzyme of the MEP pathway, structurally resembles (S)-4,5-dihydroxy-2,3-pentanedione, a signal molecule implied in bacterial cell communication. In this review, we shed light on the diversity of potential uses of the MEP pathway in antibacterial therapies, starting with an overview of the antibacterials developed for each of its enzymes. Then, we provide insight into HMBPP, its synthetic analogs, and their prodrugs. Finally, we discuss the potential contribution of the MEP pathway to quorum sensing mechanisms. The MEP pathway, providing simultaneously antibacterial drug targets and potent immunostimulants, coupled with its potential role in bacterial cell-cell communication, opens new therapeutic perspectives.
Keywords: MEP pathway; antibacterial drug; immunostimulant; isoprenoids; laurencione; phosphoantigen; prodrug; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
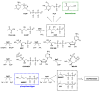
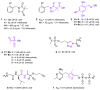

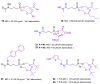
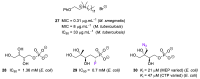




References
-
- Cane D.E., Du S., Robinson J.K., Hsiung Y., Spenser I.D. Biosynthesis of vitamin B6: Enzymatic conversion of 1-deoxy-ᴅ-xylulose-5-phosphate to pyridoxol phosphate. J. Am. Chem. Soc. 1999;121:7722–7723. doi: 10.1021/ja9914947. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

