Towards Antibiotic Synthesis in Continuous-Flow Processes
- PMID: 36771086
- PMCID: PMC9919330
- DOI: 10.3390/molecules28031421
Towards Antibiotic Synthesis in Continuous-Flow Processes
Abstract
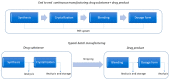
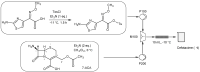
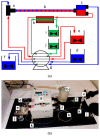
Continuous-flow chemistry has become a mainstream process and a notable trend among emerging technologies for drug synthesis. It is routinely used in academic and industrial laboratories to generate a wide variety of molecules and building blocks. The advantages it provides, in terms of safety, speed, cost efficiency and small-equipment footprint compared to analog batch processes, have been known for some time. What has become even more important in recent years is its compliance with the quality objectives that are required by drug-development protocols that integrate inline analysis and purification tools. There can be no doubt that worldwide government agencies have strongly encouraged the study and implementation of this innovative, sustainable and environmentally friendly technology. In this brief review, we list and evaluate the development and applications of continuous-flow processes for antibiotic synthesis. This work spans the period of 2012-2022 and highlights the main cases in which either active ingredients or their intermediates were produced under continuous flow. We hope that this manuscript will provide an overview of the field and a starting point for a deeper understanding of the impact of flow chemistry on the broad panorama of antibiotic synthesis.
Keywords: antibiotics; continuous process; drug synthesis; flow chemistry; industrial application; miniaturization; process control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
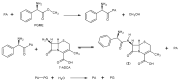
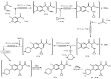
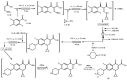

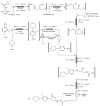


References
-
- Baraldi P.T., Hessel V. Micro reactor and flow chemistry for industrial applications in drug discovery and development. Green Process. Synth. 2012;1:149–167. doi: 10.1515/gps-2012-0008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

