Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19
- PMID: 36771194
- PMCID: PMC9920365
- DOI: 10.3390/nu15030488
Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19
Abstract
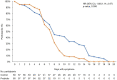
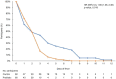
Spore-based Bacillus probiotic treatment improves intestinal health. The intestinal microbiota influences both the innate and adaptive immune responses. As such, the influence of ongoing spore-based probiotic treatment (five probiotic strains of Bacillus) on the clinical outcomes of mild COVID-19 was evaluated in this retrospective, observational study. Demographics, medical history, probiotic use, and COVID-19 symptom information were collected. The study included 120 patients with a PCR-confirmed SARS-CoV-2 infection and mild COVID-19 symptoms. The probiotic group (n = 60) comprised patients with ongoing probiotic treatment (≥1 month); the control group comprised patients not taking probiotics (n = 60). The primary outcome was time to symptom resolution; secondary outcomes included time to fever resolution and presence of digestive symptoms. The probiotic group had a significantly shorter time to symptom resolution (mean (95% confidence interval) days: control group, 8.48 (6.56, 10.05); probiotic group, 6.63 (5.56; 6.63); p = 0.003) and resolution of fever (control group, 2.67 (1.58, 3.61); probiotic group, 1.48 (1.21, 2.03); p < 0.001). More patients in the probiotic group (n = 53) than in the control group (n = 34) did not have digestive symptoms (p < 0.001). Among adults with mild COVID-19, participants receiving ongoing probiotic treatment had a shorter clinical course, and fewer had digestive symptoms compared with those not taking probiotics.
Keywords: Bacillus; COVID-19; SARS-CoV-2; fever in COVID-19 infection; gastrointestinal COVID-19 symptoms; gut microbiota; gut–lung axis; immunomodulation; probiotics; time to symptom resolution.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures


Similar articles
-
Alteration of the Gut-Lung Axis After Severe COVID-19 Infection and Modulation Through Probiotics: A Randomized, Controlled Pilot Study.Nutrients. 2024 Nov 8;16(22):3840. doi: 10.3390/nu16223840. Nutrients. 2024. PMID: 39599626 Free PMC article. Clinical Trial.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
A probiotic treatment increases the immune response induced by the nasal delivery of spore-adsorbed TTFC.Microb Cell Fact. 2020 Feb 19;19(1):42. doi: 10.1186/s12934-020-01308-1. Microb Cell Fact. 2020. PMID: 32075660 Free PMC article.
-
[Fermented milk and probiotic foods are an important part of population diet during SARS-CoV-2 pandemic].Vopr Pitan. 2022;91(1):86-97. doi: 10.33029/0042-8833-2022-91-1-86-97. Epub 2022 Jan 11. Vopr Pitan. 2022. PMID: 35298107 Review. Russian.
-
Probiotics-Derived Peptides and Their Immunomodulatory Molecules Can Play a Preventive Role Against Viral Diseases Including COVID-19.Probiotics Antimicrob Proteins. 2021 Jun;13(3):611-623. doi: 10.1007/s12602-020-09727-7. Epub 2020 Nov 23. Probiotics Antimicrob Proteins. 2021. PMID: 33226581 Free PMC article. Review.
Cited by
-
Alterations in microbiota of patients with COVID-19: implications for therapeutic interventions.MedComm (2020). 2024 Mar 15;5(4):e513. doi: 10.1002/mco2.513. eCollection 2024 Apr. MedComm (2020). 2024. PMID: 38495122 Free PMC article. Review.
-
Efficacy of a Multistrain Synbiotic Treatment in Acute and Post-Acute COVID-19 Patients: A Double-Blind, Placebo-Controlled Randomized Trial.Microorganisms. 2024 Jul 16;12(7):1443. doi: 10.3390/microorganisms12071443. Microorganisms. 2024. PMID: 39065211 Free PMC article.
-
Probiotic Therapy of Gastrointestinal Symptoms During COVID-19 Infection: A Randomized, Double-Blind, Placebo-Controlled, Remote Study.Nutrients. 2024 Nov 20;16(22):3970. doi: 10.3390/nu16223970. Nutrients. 2024. PMID: 39599756 Free PMC article. Clinical Trial.
-
Gut Microbiota Dysbiosis in COVID-19: Modulation and Approaches for Prevention and Therapy.Int J Mol Sci. 2023 Jul 31;24(15):12249. doi: 10.3390/ijms241512249. Int J Mol Sci. 2023. PMID: 37569625 Free PMC article. Review.
References
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 16 January 2023)]. Available online: https://covid19.who.int/#:~:text=Globally%2C%20as%20of%204%3A54pm,vaccin....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

