Limosilactobacillus reuteri DSM 17938-Containing Infant Formulas and the Associations with Gastrointestinal Tolerance: A Cross-Sectional Observational Study
- PMID: 36771237
- PMCID: PMC9919438
- DOI: 10.3390/nu15030530
Limosilactobacillus reuteri DSM 17938-Containing Infant Formulas and the Associations with Gastrointestinal Tolerance: A Cross-Sectional Observational Study
Abstract
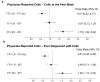
Limosilactobacillus (L.; previously Lactobacillus) reuteri has been shown to influence gastrointestinal (GI) tolerance. This study was a secondary analysis of GI tolerance data from a multi-country, cross-sectional, observational study in healthy infants using the validated Infant Gastrointestinal Symptom Questionnaire (IGSQ) and a gut comfort questionnaire. Breastfed infants (BFI; n = 760) were compared to formula-fed infants receiving either L. reuteri-containing formula (FFI + LR; n = 470) or standard formula without any probiotic or prebiotic (FFI-Std; n = 501). The IGSQ composite scores (adjusted mean ± SE) in FFI + LR (22.17 ± 0.39) was significantly lower than in FFI-Std (23.41 ± 0.37) and similar to BFI (22.34 ± 0.30;), indicating better GI tolerance in FFI + LR than in FFI-Std. Compared with FFI-Std, FFI + LR had lower reports of difficulty in passing stools (11% vs. 22%; adjusted-odds ratio (OR) (95%CI) = 0.46 (0.31-0.68)), fewer hard stools (mean difference = -0.12 (-0.21, -0.02)) and less physician-confirmed colic (OR = 0.61 (0.45-0.82)), and similar to BFI. Parent-reported crying time (mean difference = -0.15 (-0.28, -0.01)), frequency of spitting-up/vomiting (mean difference = -0.18 (-0.34, -0.03)), volume of spit-up (mean difference = -0.20 (-0.32, -0.08)) and fussiness due to spitting-up/vomiting (mean difference = -0.17 (-0.29, -0.05)) were lower in FFI + LR versus FFI-Std and similar to BFI. In this study, L. reuteri-containing formula was associated with improved digestive tolerance and behavioral patterns.
Keywords: colic; gastrointestinal tolerance; infant; infant formula; nutrition; probiotics.
Conflict of interest statement
L. Lavalle, N. Sauvageot, CI. Cercamondi, D. Egli, I. Jankovic are or were current employees of Société des Produits Nestlé S.A., Vevey, Switzerland. Y. Vandenplas has participated as a clinical investigator and/or advisory board member and/or consultant and/or speaker for Abbott Nutrition, Ausnutria, Biogaia, By Heart, CHR Hansen, Danone, ELSE Nutrition, Friesland Campina, Nestle Health Science, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, Pileje, United Pharmaceuticals (Novalac), Yakult and Wyeth.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

