Bacillus amyloliquifaciens- Supplemented Camel Milk Suppresses Neuroinflammation of Autoimmune Encephalomyelitis in a Mouse Model by Regulating Inflammatory Markers
- PMID: 36771257
- PMCID: PMC9921734
- DOI: 10.3390/nu15030550
Bacillus amyloliquifaciens- Supplemented Camel Milk Suppresses Neuroinflammation of Autoimmune Encephalomyelitis in a Mouse Model by Regulating Inflammatory Markers
Abstract
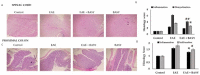
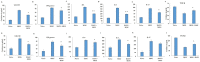
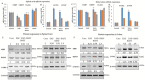
Multiple sclerosis (MS), a distinct autoimmune neuroinflammatory disorder, affects millions of people worldwide, including Saudi Arabia. Changes in the gut microbiome are linked to the development of neuroinflammation via mechanisms that are not fully understood. Prebiotics and probiotics in camel milk that has been fermented have a variety of health benefits. In this study, Bacillus amyloliquefaciens-supplemented camel milk (BASY) was used to assess its preventive effect on MS symptoms in a myelin oligodendrocyte glycoprotein (MOG)-immunized C57BL6J mice model. To this end, MOG-induced experimental autoimmune encephalomyelitis (EAE) was established and the level of disease index, pathological scores, and anti-inflammatory markers of BASY-treated mice using macroscopic and microscopic examinations, qPCR and immunoblot were investigated. The results demonstrate that BASY significantly reduced the EAE disease index, increased total microbial load (2.5 fold), and improved the levels of the short-chain fatty acids propionic, butyric and caproic acids in the diseased mice group. Additionally, myeloperoxidase (MPO) proinflammatory cytokines (IL-1β, IL-6, IL-17, TNF-α) and anti-inflammatory cytokines (TGF-β) were regulated by BASY treatment. Significant suppression of MPO and VCAM levels were noticed in the BASY-treated group (from 168 to 111 µM and from 34 to 27 pg/mL, respectively), in comparison to the EAE group. BASY treatment significantly reduced the expression of inflammatory cytokines, inflammatory progression related transcripts, and inflammatory progression protein markers. In conclusion, BASY significantly reduced the symptoms of EAE mice and may be used to develop a probiotic-based diet to promote host gut health. The cumulative findings of this study confirm the significant neuroprotection of BASY in the MOG-induced mice model. They could also suggest a novel approach to the treatment of MS-associated disorders.
Keywords: Bacillus amyloliquifaciens; VCAM signals; fermented camel's milk; multiple sclerosis; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model.Nutrients. 2022 May 8;14(9):1967. doi: 10.3390/nu14091967. Nutrients. 2022. PMID: 35565934 Free PMC article.
-
Probiotic-Fermented Camel Milk Attenuates Neurodegenerative Symptoms via SOX5/miR-218 Axis Orchestration in Mouse Models.Pharmaceuticals (Basel). 2023 Feb 25;16(3):357. doi: 10.3390/ph16030357. Pharmaceuticals (Basel). 2023. PMID: 36986457 Free PMC article.
-
Translocator Protein Ligand PIGA1138 Reduces Disease Symptoms and Severity in Experimental Autoimmune Encephalomyelitis Model of Primary Progressive Multiple Sclerosis.Mol Neurobiol. 2022 Mar;59(3):1744-1765. doi: 10.1007/s12035-022-02737-2. Epub 2022 Jan 11. Mol Neurobiol. 2022. PMID: 35018577
-
The importance of gut-brain axis and use of probiotics as a treatment strategy for multiple sclerosis.Mult Scler Relat Disord. 2023 Mar;71:104547. doi: 10.1016/j.msard.2023.104547. Epub 2023 Feb 2. Mult Scler Relat Disord. 2023. PMID: 36805171 Review.
-
Advantages and limitations of experimental autoimmune encephalomyelitis in breaking down the role of the gut microbiome in multiple sclerosis.Front Mol Neurosci. 2022 Nov 4;15:1019877. doi: 10.3389/fnmol.2022.1019877. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36407764 Free PMC article. Review.
Cited by
-
A Perspective on Oral Immunotherapeutic Tools and Strategies for Autoimmune Disorders.Vaccines (Basel). 2023 May 27;11(6):1031. doi: 10.3390/vaccines11061031. Vaccines (Basel). 2023. PMID: 37376420 Free PMC article. Review.
-
The potential effectiveness of probiotics in reducing multiple sclerosis progression in preclinical and clinical studies: A worldwide systematic review and meta-analysis.PLoS One. 2025 Apr 24;20(4):e0319755. doi: 10.1371/journal.pone.0319755. eCollection 2025. PLoS One. 2025. PMID: 40273120 Free PMC article.
-
Attenuation of Immunogenicity in MOG-Induced Oligodendrocytes by the Probiotic Bacterium Lactococcus Sp. PO3.Medicina (Kaunas). 2023 Sep 27;59(10):1731. doi: 10.3390/medicina59101731. Medicina (Kaunas). 2023. PMID: 37893449 Free PMC article.
-
Bacterial live therapeutics for human diseases.Mol Syst Biol. 2024 Dec;20(12):1261-1281. doi: 10.1038/s44320-024-00067-0. Epub 2024 Oct 23. Mol Syst Biol. 2024. PMID: 39443745 Free PMC article. Review.
-
Bacillus subtilis PM5 from Camel Milk Boosts Chicken Immunity and Abrogates Salmonella entertitidis Infections.Microorganisms. 2023 Jun 30;11(7):1719. doi: 10.3390/microorganisms11071719. Microorganisms. 2023. PMID: 37512891 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

