Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway
- PMID: 36771283
- PMCID: PMC9918937
- DOI: 10.3390/nu15030575
Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway
Abstract
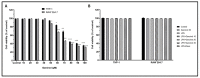
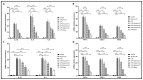
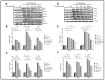
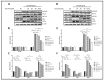
Garcinia is a significant medicinal plant with many beneficial phytoconstituents, including garcinol. This study investigated the anti-inflammatory effect of garcinol isolated from Garcinia dulcis fruit in LPS-activated THP-1 and Raw 264.7 macrophages. The results demonstrated that the low concentration of garcinol did not alter cell viability. Furthermore, co-incubation of garcinol with LPS inhibited the production of pro-inflammatory cytokines, including TNF-α, IL-8, IL-6, IL-1β, and pro-inflammatory mediators, including iNOS and COX-2 at the mRNA and protein expression levels. Garcinol also decreased the secretion of TNF-α, IL-6, IL-1β, PGE2, and NO. Moreover, the anti-inflammatory effects involved an alteration in the NF-κB signaling pathway. Downregulation of pIKKα/β, pIκBα, and pNF-κB was observed, hence reducing the translocation of pNF-κB from the cytosol into the nucleus, which subsequently decreased the production of pro-inflammatory molecules. Therefore, garcinol isolated from Garcinia dulcis is a potential candidate as an anti-inflammatory agent for inflammation-related disease treatment.
Keywords: Garcinia dulcis; NF-κB; RAW 264.7; THP-1; garcinol; inflammation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
DXXK exerts anti-inflammatory effects by inhibiting the lipopolysaccharide-induced NF-κB/COX-2 signalling pathway and the expression of inflammatory mediators.J Ethnopharmacol. 2016 Feb 3;178:199-208. doi: 10.1016/j.jep.2015.11.016. Epub 2015 Nov 10. J Ethnopharmacol. 2016. PMID: 26571085
-
Aquaporins (AQPs) as a marker in the physiology of inflammation and its interaction studies with garcinol.Inflammopharmacology. 2024 Apr;32(2):1575-1592. doi: 10.1007/s10787-023-01412-9. Epub 2024 Jan 24. Inflammopharmacology. 2024. PMID: 38267609
-
Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages.BMC Complement Altern Med. 2014 Mar 15;14:101. doi: 10.1186/1472-6882-14-101. BMC Complement Altern Med. 2014. PMID: 24628870 Free PMC article.
-
Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages.Int Immunopharmacol. 2016 Nov;40:146-155. doi: 10.1016/j.intimp.2016.08.021. Epub 2016 Sep 1. Int Immunopharmacol. 2016. PMID: 27591413
-
Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-κB signaling pathway.J Ethnopharmacol. 2016 May 13;183:159-165. doi: 10.1016/j.jep.2016.01.018. Epub 2016 Jan 19. J Ethnopharmacol. 2016. PMID: 26806575
Cited by
-
Cardiovascular Protective Effect of Garcinia dulcis Flower Acetone Extract in 2-Kidney-1-Clip Hypertensive Rats.Adv Pharmacol Pharm Sci. 2024 Feb 29;2024:9916598. doi: 10.1155/2024/9916598. eCollection 2024. Adv Pharmacol Pharm Sci. 2024. PMID: 38455637 Free PMC article.
-
Plant-Based Functional Foods from Borneo.Nutrients. 2025 Jan 7;17(2):200. doi: 10.3390/nu17020200. Nutrients. 2025. PMID: 39861330 Free PMC article. Review.
-
Garcinia dulcis Extract Alleviates Inflammation in Kidney and Liver of the 2-Kidney-1-Clip Hypertensive rat.J Evid Based Integr Med. 2024 Jan-Dec;29:2515690X241244845. doi: 10.1177/2515690X241244845. J Evid Based Integr Med. 2024. PMID: 38613379 Free PMC article.
-
The Role of T-Cadherin (CDH13) in Treatment Options with Garcinol in Melanoma.Cancers (Basel). 2024 May 12;16(10):1853. doi: 10.3390/cancers16101853. Cancers (Basel). 2024. PMID: 38791932 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

