Endogenous n-3 PUFAs Improve Non-Alcoholic Fatty Liver Disease through FFAR4-Mediated Gut-Liver Crosstalk
- PMID: 36771292
- PMCID: PMC9919706
- DOI: 10.3390/nu15030586
Endogenous n-3 PUFAs Improve Non-Alcoholic Fatty Liver Disease through FFAR4-Mediated Gut-Liver Crosstalk
Abstract
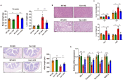
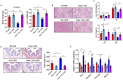
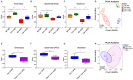
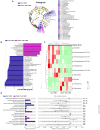
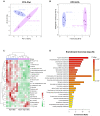
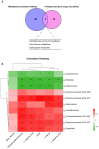
The gut-liver axis plays a key role in the development and progression of non-alcoholic fatty liver disease (NAFLD). Due to the complexity and incomplete understanding of the cross-talk between the gut and liver, effective therapeutic targets are largely unknown. Free fatty acid receptors (FFARs) may bridge the cross-talk between the gut and liver. FFAR4 has received considerable attention due to its important role in lipid metabolism. However, the role of FFAR4 in this cross talk in NAFLD remains unclear. In this study, mice with high endogenous n-3 PUFAs but FFAR4 deficiency were generated by crossbreeding Fat-1 and FFAR4 knockout mice. FFAR4 deficiency blocked the protective effects of high endogenous n-3 PUFAs on intestinal barrier dysfunction and hepatic steatosis. In addition, FFAR4 deficiency decreased gut microbiota diversity and enriched Rikenella, Anaerotruncus, and Enterococcus, and reduced Dubosiella, Ruminococcaceae UCG-010, Ruminococcaceae UCG-014, Coriobacteriaceae UCG-002, Faecalibaculum, Ruminococcaceae UCG-009, and Akkermansia. Notably, FFAR4 deficiency co-regulated pantothenic acid and CoA biosynthesis, β-alanine metabolism, and sphingolipid metabolism pathways in the gut and liver, potentially associated with the aggravation of NAFLD. Together, the beneficial effects of n-3 PUFAs on the gut and liver were mediated by FFAR4, providing insights on the role of FFAR4 in the treatment of NAFLD through the gut-liver axis.
Keywords: free fatty acid receptor 4; gut–liver axis; n-3 polyunsaturated fatty acids; non-alcoholic fatty liver disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A High-Fat Diet Increases the Characteristics of Gut Microbial Composition and the Intestinal Damage Associated with Non-Alcoholic Fatty Liver Disease.Int J Mol Sci. 2023 Nov 24;24(23):16733. doi: 10.3390/ijms242316733. Int J Mol Sci. 2023. PMID: 38069055 Free PMC article.
-
The Ameliorating Effects of n-3 Polyunsaturated Fatty Acids on Liver Steatosis Induced by a High-Fat Methionine Choline-Deficient Diet in Mice.Int J Mol Sci. 2023 Dec 7;24(24):17226. doi: 10.3390/ijms242417226. Int J Mol Sci. 2023. PMID: 38139055 Free PMC article.
-
The Influence of the FFAR4 Agonist TUG-891 on Liver Steatosis in ApoE-Knockout Mice.Cardiovasc Drugs Ther. 2024 Aug;38(4):667-678. doi: 10.1007/s10557-023-07430-7. Epub 2023 Jan 27. Cardiovasc Drugs Ther. 2024. PMID: 36705799 Free PMC article.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
[Involvement of the Free Fatty Acid Receptor GPR120/FFAR4 in the Development of Nonalcoholic Steatohepatitis].Yakugaku Zasshi. 2019;139(9):1169-1175. doi: 10.1248/yakushi.19-00011-4. Yakugaku Zasshi. 2019. PMID: 31474633 Review. Japanese.
Cited by
-
Study on characteristics of gut flora composition of pregnant women with preeclampsia.BMC Pregnancy Childbirth. 2025 May 31;25(1):636. doi: 10.1186/s12884-025-07760-4. BMC Pregnancy Childbirth. 2025. PMID: 40450263 Free PMC article.
-
FFAR4 activation inhibits lung adenocarcinoma via blocking respiratory chain complex assembly associated mitochondrial metabolism.Cell Mol Biol Lett. 2024 Jan 19;29(1):17. doi: 10.1186/s11658-024-00535-3. Cell Mol Biol Lett. 2024. PMID: 38243188 Free PMC article.
-
Gut-Liver Axis: The Role of Intestinal Microbiota and Their Metabolites in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease.Gut Liver. 2025 Jul 15;19(4):479-507. doi: 10.5009/gnl240539. Epub 2025 May 8. Gut Liver. 2025. PMID: 40336226 Free PMC article. Review.
-
The influence of perilipin 5 deficiency on gut microbiome profiles in murine metabolic dysfunction-associated fatty liver disease (MAFLD) and MAFLD-hepatocellular carcinoma.Front Cell Infect Microbiol. 2024 Oct 14;14:1443654. doi: 10.3389/fcimb.2024.1443654. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39469452 Free PMC article.
-
Fat-1 Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease and Atherosclerosis through Promoting the Nuclear Localization of PPARα in Hamsters.Research (Wash D C). 2025 Mar 6;8:0577. doi: 10.34133/research.0577. eCollection 2025. Research (Wash D C). 2025. PMID: 40052160 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

