Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases
- PMID: 36771677
- PMCID: PMC9921826
- DOI: 10.3390/plants12030594
Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases
Abstract
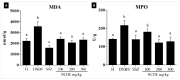
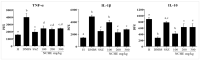
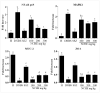
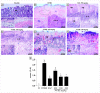
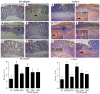
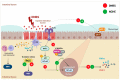
Phenolic compounds have been scientifically recognized as beneficial to intestinal health. The cactus Nopalea cochenillifera, used as anti-inflammatory in traditional medicine, is a rich source of these bioactive compounds. The present study aimed to investigate the phytochemical profile of N. cochenillifera extract and evaluate its acute toxicity and anti-inflammatory effect on 2,4-dinitrobenzenesulfonic acid (DNBS)-induced colitis in rats. The total phenolic content per gram of dry extract was 67.85 mg. Through HPLC-IES-MSn, a total of 25 compounds such as saccharides, organic acids, phenolic acids and flavonoids were characterized. The dose of 2000 mg/kg of extract by an oral route showed no signs of toxicity, mortality or significant changes in biochemical and hematological parameters. Regarding intestinal anti-inflammatory effects, animals were treated with three different doses of extract or sulfasalazine. Macroscopic analysis of the colon indicated that the extract decreased the disease activity index. Levels of IL-1β and TNF-α decreased, IL-10 increased and MDA and MPO enzyme levels decreased when compared with the control group. In addition, a down-regulation of MAPK1/ERK2 and NF-κB p65 pathway markers in colon tissue was observed. The epithelial integrity was improved according to histopathological and immunohistological analysis. Thus, the extract provided strong preclinical evidence of being effective in maintaining the remission of colitis.
Keywords: Cactaceae; colitis; flavonoids; functional foods; herbal medicines; inflammatory chronic diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Alatab S., Sepanlou S.G., Ikuta K., Vahedi H., Bisignano C., Safiri S., Sadeghi A., Nixon M.R., Abdoli A., Abolhassani H., et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. - DOI - PMC - PubMed
-
- Khan S., Ayyagari R., Shah R., Herrera H., Swaroop P. The Use of Oral Complementary and Alternative Medicine in Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2012;107:S684–S685. doi: 10.14309/00000434-201210001-01689. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

