Continuous Phosphate Removal and Recovery Using a Calcium Silicate Hydrate Composite Monolithic Cryogel Column
- PMID: 36771839
- PMCID: PMC9921571
- DOI: 10.3390/polym15030539
Continuous Phosphate Removal and Recovery Using a Calcium Silicate Hydrate Composite Monolithic Cryogel Column
Abstract
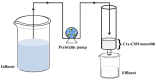
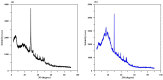
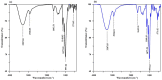
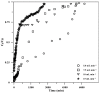
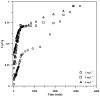
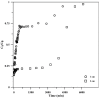
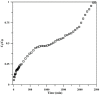
Toward the development of a practical and green approach for removing phosphate from water, a monolithic cryogel based on starch and calcium silicate hydrate (Cry-CSH) was employed as a phosphate adsorbent in a continuous flow system for the first time. The influence of flow rate, initial phosphate concentration, and adsorbent height on the adsorption efficiency was investigated. As the rate of flow and the initial concentration of phosphate increased, the total quantity of adsorbed phosphate dropped; however, the performance of the column was greatly enhanced by an increase in adsorbent height. The experimental data fit the Adams-Bohart model better than the Thomas and Yoon-Nelson models at the beginning of the adsorption process. To evaluate its applicability, the continuous flow system based on the monolithic Cry-CSH column was applied for the removal of phosphate from the discharge effluent of the Patong Municipality Wastewater Treatment Plant (Phuket, Thailand), achieving an excellent total adsorption of 94.61%.
Keywords: calcium silicate hydrate; continuous flow adsorption; phosphate removal; starch cryogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Continuous-flow phosphate removal using Cry-Ca-COS Monolith: Insights from dynamic adsorption modeling.Water Res X. 2024 Dec 13;27:100296. doi: 10.1016/j.wroa.2024.100296. eCollection 2025 May 1. Water Res X. 2024. PMID: 39811253 Free PMC article.
-
Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column.Polymers (Basel). 2023 Oct 4;15(19):3989. doi: 10.3390/polym15193989. Polymers (Basel). 2023. PMID: 37836037 Free PMC article.
-
Recovering Phosphate from Complex Wastewater Using Macroporous Cryogel Composited Calcium Silicate Hydrate Nanoparticles.Molecules. 2023 Dec 31;29(1):228. doi: 10.3390/molecules29010228. Molecules. 2023. PMID: 38202812 Free PMC article.
-
Removal and recovery of phosphate using a novel calcium silicate hydrate composite starch cryogel.J Environ Manage. 2022 Jan 1;301:113923. doi: 10.1016/j.jenvman.2021.113923. Epub 2021 Oct 8. J Environ Manage. 2022. PMID: 34634722
-
Fixed-Bed Column Technique for the Removal of Phosphate from Water Using Leftover Coal.Materials (Basel). 2021 Sep 22;14(19):5466. doi: 10.3390/ma14195466. Materials (Basel). 2021. PMID: 34639864 Free PMC article.
Cited by
-
Continuous-flow phosphate removal using Cry-Ca-COS Monolith: Insights from dynamic adsorption modeling.Water Res X. 2024 Dec 13;27:100296. doi: 10.1016/j.wroa.2024.100296. eCollection 2025 May 1. Water Res X. 2024. PMID: 39811253 Free PMC article.
-
Adsorption of Phosphate by Two-Step Synthesis of Ceramsite from Electrolytic Manganese Residue/Dredged Sludge.Materials (Basel). 2024 Feb 17;17(4):939. doi: 10.3390/ma17040939. Materials (Basel). 2024. PMID: 38399190 Free PMC article.
-
Remediation of lead toxicity with waste-bio materials from aqueous solutions in fixed-bed column using response surface methodology.Heliyon. 2024 Jul 26;10(15):e35173. doi: 10.1016/j.heliyon.2024.e35173. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39166046 Free PMC article.
-
Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column.Polymers (Basel). 2023 Oct 4;15(19):3989. doi: 10.3390/polym15193989. Polymers (Basel). 2023. PMID: 37836037 Free PMC article.
-
Recovering Phosphate from Complex Wastewater Using Macroporous Cryogel Composited Calcium Silicate Hydrate Nanoparticles.Molecules. 2023 Dec 31;29(1):228. doi: 10.3390/molecules29010228. Molecules. 2023. PMID: 38202812 Free PMC article.
References
-
- Kuwahara Y., Yamashita H. Phosphate removal from aqueous solutions using calcium silicate hydrate prepared from blast furnace slag. ISIJ Int. 2017;57:1657–1664. doi: 10.2355/isijinternational.ISIJINT-2017-123. - DOI
-
- Ayoub G.M., Kalinian H., Zayyat R. Efficient phosphate removal from contaminated water using functional raw dolomite powder. SN Appl. Sci. 2019;1:802. doi: 10.1007/s42452-019-0833-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

