Fluorescent Molecularly Imprinted Polymers Loaded with Avenanthramides for Inhibition of Advanced Glycation End Products
- PMID: 36771840
- PMCID: PMC9920636
- DOI: 10.3390/polym15030538
Fluorescent Molecularly Imprinted Polymers Loaded with Avenanthramides for Inhibition of Advanced Glycation End Products
Abstract
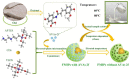
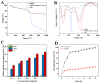
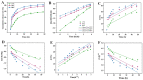
Encapsulating bioactive avenanthramides (AVAs) in carriers to respond to the environmental changes of food thermal processing allows the controlled release of AVAs for the effective inhibition of biohazards. In this study, fluorescent molecular imprinted polymers (FMIPs) loaded with AVAs were prepared by reverse microemulsion. The fluorescent signal was generated by carbon dots (CDs), which were derived from oat bran to determine the load of AVAs. The FMIPs were uniformly spherical in appearance and demonstrated favorable properties, such as thermal stability, protection of AVAs against photodegradation, high encapsulation efficiency, and effective scavenging of free radicals. After consideration of the different kinetics models, the release of AVAs from the FMIPs matched the Weibull model and followed a Fickian diffusion mechanism. The FMIPs exhibited good inhibition of pyrraline in a simulated casein-ribose system and in milk samples, indicating the release of AVAs could inhibit the generation of pyrraline.
Keywords: avenanthramides; molecular imprinted polymers; pyrraline; release; reverse microemulsion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Molecularly imprinted thermosensitive probe based on fluorescent advanced glycation end products to detect α-dicarbonyl compounds and inhibit pyrraline formation.Anal Bioanal Chem. 2023 Aug;415(20):5011-5021. doi: 10.1007/s00216-023-04787-4. Epub 2023 Jun 21. Anal Bioanal Chem. 2023. PMID: 37341783
-
Inhibitory Mechanism of Advanced Glycation End-Product Formation by Avenanthramides Derived from Oats through Scavenging the Intermediates.Foods. 2022 Jun 20;11(12):1813. doi: 10.3390/foods11121813. Foods. 2022. PMID: 35742012 Free PMC article.
-
Quantitative Analysis and Anti-inflammatory Activity Evaluation of the A-Type Avenanthramides in Commercial Sprouted Oat Products.J Agric Food Chem. 2020 Nov 18;68(46):13068-13075. doi: 10.1021/acs.jafc.9b06812. Epub 2019 Dec 16. J Agric Food Chem. 2020. PMID: 31841331
-
Overview of the Anticancer Profile of Avenanthramides from Oat.Int J Mol Sci. 2019 Sep 13;20(18):4536. doi: 10.3390/ijms20184536. Int J Mol Sci. 2019. PMID: 31540249 Free PMC article. Review.
-
Glycation in food and metabolic transit of dietary AGEs (advanced glycation end-products): studies on the urinary excretion of pyrraline.Biochem Soc Trans. 2003 Dec;31(Pt 6):1383-5. doi: 10.1042/bst0311383. Biochem Soc Trans. 2003. PMID: 14641068 Review.
References
-
- Peterson D.M., Dimberg L.H. Avenanthramide concentrations and hydroxycinnamoyl-CoA:hydroxyanthranilate N-hydroxycinnamoyltransferase activities in developing oats. J. Cereal Sci. 2008;47:101–108. doi: 10.1016/j.jcs.2007.02.007. - DOI
LinkOut - more resources
Full Text Sources

