Fabrication of Sustained Release Curcumin-Loaded Solid Lipid Nanoparticles (Cur-SLNs) as a Potential Drug Delivery System for the Treatment of Lung Cancer: Optimization of Formulation and In Vitro Biological Evaluation
- PMID: 36771843
- PMCID: PMC9918916
- DOI: 10.3390/polym15030542
Fabrication of Sustained Release Curcumin-Loaded Solid Lipid Nanoparticles (Cur-SLNs) as a Potential Drug Delivery System for the Treatment of Lung Cancer: Optimization of Formulation and In Vitro Biological Evaluation
Abstract
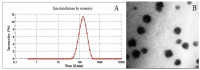
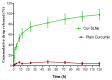
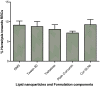
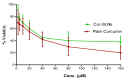
The goal of current research was to develop a new form of effective drug, curcumin-loaded solid lipid nanoparticles (Cur-SLNs) and test its efficacy in the treatment of lung cancer. Different batches of SLNs were prepared by the emulsification-ultrasonication method. For the optimization of formulation, each batch was evaluated for particle size, polydispersity index (PI), zeta potential (ZP), entrapment efficiency (EE) and drug loading (DL). The formulation components and process parameters largely affected the quality of SLNs. The SLNs obtained with particle size, 114.9 ± 1.36 nm; PI, 0.112 ± 0.005; ZP, -32.3 ± 0.30 mV; EE, 69.74 ± 2.03%, and DL, 0.81 ± 0.04% was designated as an optimized formulation. The formulation was freeze-dried to remove excess water to improve the physical stability. Freeze-dried Cur-SLNs showed 99.32% of drug release and demonstrated a burst effect trailed by sustained release up to 120 h periods. The erythrocyte toxicity study of Cur-SLNs and its components demonstrated moderate hemolytic potential towards red blood cells (RBCs). The cytotoxic potential of the formulation and plain curcumin was estimated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against A549 cell line. After 48 h of incubation, Cur-SLNs demonstrated more cytotoxicity (IC50 = 26.12 ± 1.24 µM) than plain curcumin (IC50 = 35.12 ± 2.33 µM). Moreover, the cellular uptake of curcumin was found to be significantly higher from Cur-SLNs (682.08 ± 6.33 ng/µg) compared to plain curcumin (162.4 ± 4.2 ng/µg). Additionally, the optimized formulation was found to be stable over the period of 90 days of storage. Hence, curcumin-loaded SLNs can be prepared using the proposed cost effective method, and can be utilized as an effective drug delivery system for the treatment of lung cancer, provided in vivo studies warrant a similar outcome.
Keywords: cellular uptake; curcumin; cytotoxicity; erythrocyte toxicity; lung cancer; solid lipid nanoparticle; solubility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Development of Curcumin-Loaded Solid Lipid Nanoparticles Utilizing Glyceryl Monostearate as Single Lipid Using QbD Approach: Characterization and Evaluation of Anticancer Activity Against Human Breast Cancer Cell Line.Curr Drug Deliv. 2018;15(9):1271-1283. doi: 10.2174/1567201815666180503120113. Curr Drug Deliv. 2018. PMID: 29732970
-
Preparation of Curcumin Solid Lipid Nanoparticles Loaded with Flower-Shaped Lactose for Lung Inhalation and Preliminary Evaluation of Cytotoxicity In Vitro.Evid Based Complement Alternat Med. 2021 Oct 28;2021:4828169. doi: 10.1155/2021/4828169. eCollection 2021. Evid Based Complement Alternat Med. 2021. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 13;2023:9809240. doi: 10.1155/2023/9809240. PMID: 34745284 Free PMC article. Retracted.
-
Naringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics.Drug Des Devel Ther. 2016 Mar 1;10:911-25. doi: 10.2147/DDDT.S97738. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27041995 Free PMC article.
-
Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds.Antioxidants (Basel). 2023 Mar 3;12(3):633. doi: 10.3390/antiox12030633. Antioxidants (Basel). 2023. PMID: 36978881 Free PMC article. Review.
-
Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems.Pharmaceutics. 2018 Oct 18;10(4):191. doi: 10.3390/pharmaceutics10040191. Pharmaceutics. 2018. PMID: 30340327 Free PMC article. Review.
Cited by
-
Lipid Nanoparticles in Lung Cancer Therapy.Pharmaceutics. 2024 May 10;16(5):644. doi: 10.3390/pharmaceutics16050644. Pharmaceutics. 2024. PMID: 38794306 Free PMC article. Review.
-
Inhalable TPGS/DPPC Micelles Coloaded with Curcumin and Icariin for Targeted Lung Cancer Therapy.ACS Omega. 2025 Apr 11;10(15):15400-15411. doi: 10.1021/acsomega.5c00008. eCollection 2025 Apr 22. ACS Omega. 2025. PMID: 40290948 Free PMC article.
-
Formulation, Optimization, and Ex vivo Permeation Study of Ritonavir-loaded Solid Lipid Nanoparticles.Curr Pharm Des. 2025;31(26):2105-2116. doi: 10.2174/0113816128329768241230071303. Curr Pharm Des. 2025. PMID: 40012287
-
Enhancing Surface Temperature Uniformity in a Liquid Silicone Rubber Injection Mold with Conformal Heating Channels.Materials (Basel). 2023 Aug 22;16(17):5739. doi: 10.3390/ma16175739. Materials (Basel). 2023. PMID: 37687431 Free PMC article.
-
Uncovering the Emerging Prospects of Lipid-based Nanoparticulate Vehicles in Lung Cancer Management: A Recent Perspective.Pharm Nanotechnol. 2025;13(1):155-170. doi: 10.2174/0122117385286781240228060152. Pharm Nanotechnol. 2025. PMID: 38468532 Review.
References
-
- Awaad A.S., Maitland D.J., Moneir S.M. New alkaloids from Casimiroa edulis fruits and their pharmacological activity. Chem. Nat. Comp. 2007;43:576–580. doi: 10.1007/s10600-007-0196-9. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

