Development of Polydiphenylamine@Electrochemically Reduced Graphene Oxide Electrode for the D-Penicillamine Sensor from Human Blood Serum Samples Using Amperometry
- PMID: 36771878
- PMCID: PMC9921737
- DOI: 10.3390/polym15030577
Development of Polydiphenylamine@Electrochemically Reduced Graphene Oxide Electrode for the D-Penicillamine Sensor from Human Blood Serum Samples Using Amperometry
Abstract
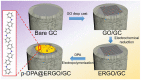
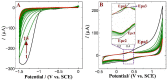
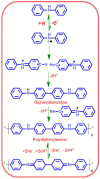
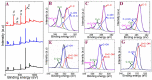
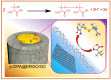
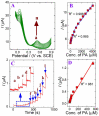
D-penicillamine (PA) is a sulfur group-containing drug prescribed for various health issues, but overdoses have adverse effects. Therefore, regular, selective, and sensitive sensing is essential to reduce the need for further treatment. In this study, diphenylamine (DPA) was electropolymerized in an aqueous acidic medium. The PA detection sensitivity, selectivity, and limit of detection were enhanced by electropolymerizing DPA on an electrochemically reduced graphene oxide (ERGO)/glassy carbon (GC) surface. The formation of p-DPA and ERGO was investigated using various techniques. The as-prepared p-DPA@ERGO/GC revealed the excellent redox-active (N-C to N=C) sites of p-DPA. The p-DPA@ERGO/GC electrode exhibited excellent electrochemical sensing ability towards PA determination because of the presence of the -NH-functional moiety and effective interactions with the -SH group of PA. The p-DPA@ERGO/GC exhibited a high surface coverage of 9.23 × 10-12 mol cm-2. The polymer-modified p-DPA@ERGO/GC electrode revealed the amperometric determination of PA concentration from the 1.4 to 541 μM wide range and the detection limit of 0.10 μM. The real-time feasibility of the developed p-DPA@ERGO/GC electrode was tested with a realistic PA finding in human blood serum samples and yielded a good recovery of 97.5-101.0%, confirming the potential suitability in bio-clinical applications.
Keywords: D-penicillamine; cyclic voltammetry; electrochemically reduced graphene oxide; electropolymerization; polydiphenylamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Potentiodynamic formation of diaminobenzene films on an electrochemically reduced graphene oxide surface: Determination of nitrite in water samples.Mater Sci Eng C Mater Biol Appl. 2018 Apr 1;85:97-106. doi: 10.1016/j.msec.2017.12.004. Epub 2017 Dec 18. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 29407162
-
Molecularly imprinted hornlike polymer@electrochemically reduced graphene oxide electrode for the highly selective determination of an antiemetic drug.Anal Chim Acta. 2021 Jan 2;1141:71-82. doi: 10.1016/j.aca.2020.10.014. Epub 2020 Oct 14. Anal Chim Acta. 2021. PMID: 33248664
-
Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow.Molecules. 2018 Aug 24;23(9):2130. doi: 10.3390/molecules23092130. Molecules. 2018. PMID: 30149513 Free PMC article.
-
Poly(caffeic acid) Redox Couple Decorated on Electrochemically Reduced Graphene Oxide for Electrocatalytic Sensing Free Chlorine in Drinking Water.Nanomaterials (Basel). 2022 Dec 28;13(1):151. doi: 10.3390/nano13010151. Nanomaterials (Basel). 2022. PMID: 36616061 Free PMC article.
-
Electropolymerized Melamine on Electrochemically Reduced Graphene Oxide: Growth Mechanistics, Electrode Processing, and Amperometric Sensing of Acyclovir.Langmuir. 2023 Mar 7;39(9):3512-3525. doi: 10.1021/acs.langmuir.3c00128. Epub 2023 Feb 23. Langmuir. 2023. PMID: 36820624
Cited by
-
Construction and Application of an Electrochemical Sensor for Determination of D-Penicillamine Based on Modified Carbon Paste Electrode.Micromachines (Basel). 2024 Jan 31;15(2):220. doi: 10.3390/mi15020220. Micromachines (Basel). 2024. PMID: 38398949 Free PMC article.
References
-
- Maziz A., Özgür E., Bergaud C., Uzun L. Progress in conducting polymers for biointerfacing and biorecognition applications. Sens. Actuators Rep. 2021;3:100035. doi: 10.1016/j.snr.2021.100035. - DOI
-
- El-Kady M.F., Shao Y., Kaner R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016;1:16033. doi: 10.1038/natrevmats.2016.33. - DOI
-
- Shamkhalichenar H., Choi J.-W. Review—Non-Enzymatic Hydrogen Peroxide Electrochemical Sensors Based on Reduced Graphene Oxide. J. Electrochem. Soc. 2020;167:037531. doi: 10.1149/1945-7111/ab644a. - DOI
-
- He B., Yan D. Au/ERGO nanoparticles supported on Cu-based metal-organic framework as a novel sensor for sensitive determination of nitrite. Food Control. 2019;103:70–77. doi: 10.1016/j.foodcont.2019.04.001. - DOI
-
- Zhang Z., Xiao F., Qian L., Xiao J., Wang S., Liu Y. Facile Synthesis of 3D MnO2–Graphene and Carbon Nanotube–Graphene Composite Networks for High-Performance, Flexible, All-Solid-State Asymmetric Supercapacitors. Adv. Energy Mater. 2014;4:1400064. doi: 10.1002/aenm.201400064. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

