Accelerating Payload Release from Complex Coacervates through Mechanical Stimulation
- PMID: 36771888
- PMCID: PMC9919863
- DOI: 10.3390/polym15030586
Accelerating Payload Release from Complex Coacervates through Mechanical Stimulation
Abstract
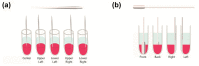
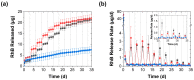
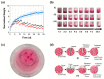
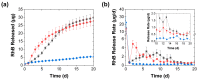
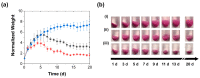
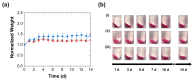
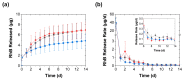
Complex coacervates formed through the association of charged polymers with oppositely charged species are often investigated for controlled release applications and can provide highly sustained (multi-day, -week or -month) release of both small-molecule and macromolecular actives. This release, however, can sometimes be too slow to deliver the active molecules in the doses needed to achieve the desired effect. Here, we explore how the slow release of small molecules from coacervate matrices can be accelerated through mechanical stimulation. Using coacervates formed through the association of poly(allylamine hydrochloride) (PAH) with pentavalent tripolyphosphate (TPP) ions and Rhodamine B dye as the model coacervate and payload, we demonstrate that slow payload release from complex coacervates can be accelerated severalfold through mechanical stimulation (akin to flavor release from a chewed piece of gum). The stimulation leading to this effect can be readily achieved through either perforation (with needles) or compression of the coacervates and, besides accelerating the release, can result in a deswelling of the coacervate phases. The mechanical activation effect evidently reflects the rupture and collapse of solvent-filled pores, which form due to osmotic swelling of the solute-charged coacervate pellets and is most pronounced in release media that favor swelling. This stimulation effect is therefore strong in deionized water (where the swelling is substantial) and only subtle and shorter-lived in phosphate buffered saline (where the PAH/TPP coacervate swelling is inhibited). Taken together, these findings suggest that mechanical activation could be useful in extending the complex coacervate matrix efficacy in highly sustained release applications where the slowly releasing coacervate-based sustained release vehicles undergo significant osmotic swelling.
Keywords: complex coacervate; controlled release; polyamine; polyelectrolyte; stimulus-responsive materials.
Conflict of interest statement
Y.L. declares financial interest in a patent on PAH/TPP coacervate use in underwater adhesion and sustained release applications. W.A.H. has no conflicts of interest to declare.
Figures
Similar articles
-
Poly(allylamine)/tripolyphosphate coacervates enable high loading and multiple-month release of weakly amphiphilic anionic drugs: an in vitro study with ibuprofen.RSC Adv. 2018 May 25;8(35):19409-19419. doi: 10.1039/c8ra02588f. eCollection 2018 May 25. RSC Adv. 2018. PMID: 35540986 Free PMC article.
-
Highly Sustained Release of Bactericides from Complex Coacervates.ACS Appl Bio Mater. 2020 Dec 21;3(12):8427-8437. doi: 10.1021/acsabm.0c00763. Epub 2020 Nov 13. ACS Appl Bio Mater. 2020. PMID: 35019614
-
Ionically Cross-Linked Polymer Networks for the Multiple-Month Release of Small Molecules.ACS Appl Mater Interfaces. 2016 Feb;8(7):4323-35. doi: 10.1021/acsami.5b10070. Epub 2016 Feb 11. ACS Appl Mater Interfaces. 2016. PMID: 26811936 Free PMC article.
-
Complex Coacervates as a Promising Vehicle for mRNA Delivery: A Comprehensive Review of Recent Advances and Challenges.Mol Pharm. 2023 Sep 4;20(9):4387-4403. doi: 10.1021/acs.molpharmaceut.3c00439. Epub 2023 Aug 10. Mol Pharm. 2023. PMID: 37561647 Review.
-
Sticky Science: Using Complex Coacervate Adhesives for Biomedical Applications.Adv Healthc Mater. 2025 Jan;14(2):e2402340. doi: 10.1002/adhm.202402340. Epub 2024 Oct 1. Adv Healthc Mater. 2025. PMID: 39352099 Free PMC article. Review.
Cited by
-
Smart coacervate microdroplets: biomimetic design, material innovations, and emerging applications in biomacromolecule delivery.Bioact Mater. 2025 Jun 10;52:244-270. doi: 10.1016/j.bioactmat.2025.06.016. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40547321 Free PMC article. Review.
References
-
- De Jong H.G.B. Complex colloid systems. In: Kruyt H.R., editor. Colloid Science. Volume II. Elsevier; Amsterdam, The Netherlands: 1949. pp. 335–432.
-
- Menger F.M., Sykes B.M. Anatomy of a coacervate. Langmuir. 1998;14:4131–4137. doi: 10.1021/la980208m. - DOI
-
- Momeni A., Filiaggi M.J. Rheology of polyphosphate coacervates. J. Rheol. 2016;60:25–34. doi: 10.1122/1.4935127. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

