Diffusion-Enhanced Förster Resonance Energy Transfer in Flexible Peptides: From the Haas-Steinberg Partial Differential Equation to a Closed Analytical Expression
- PMID: 36772006
- PMCID: PMC9919848
- DOI: 10.3390/polym15030705
Diffusion-Enhanced Förster Resonance Energy Transfer in Flexible Peptides: From the Haas-Steinberg Partial Differential Equation to a Closed Analytical Expression
Abstract
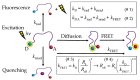
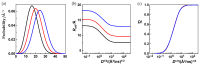
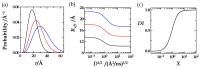
In the huge field of polymer structure and dynamics, including intrinsically disordered peptides, protein folding, and enzyme activity, many questions remain that cannot be answered by methodology based on artificial intelligence, X-ray, or NMR spectroscopy but maybe by fluorescence spectroscopy. The theory of Förster resonance energy transfer (FRET) describes how an optically excited fluorophore transfers its excitation energy through space to an acceptor moiety-with a rate that depends on the distance between donor and acceptor. When the donor and acceptor moiety are conjugated to different sites of a flexible peptide chain or any other linear polymer, the pair could in principle report on chain structure and dynamics, on the site-to-site distance distribution, and on the diffusion coefficient of mutual site-to-site motion of the peptide chain. However, the dependence of FRET on distance distribution and diffusion is not defined by a closed analytical expression but by a partial differential equation (PDE), by the Haas-Steinberg equation (HSE), which can only be solved by time-consuming numerical methods. As a second complication, time-resolved FRET measurements have thus far been deemed necessary. As a third complication, the evaluation requires a computationally demanding but indispensable global analysis of an extended experimental data set. These requirements have made the method accessible to only a few experts. Here, we show how the Haas-Steinberg equation leads to a closed analytical expression (CAE), the Haas-Steinberg-Jacob equation (HSJE), which relates a diffusion-diagnosing parameter, the effective donor-acceptor distance, to the augmented diffusion coefficient, J, composed of the diffusion coefficient, D, and the photophysical parameters that characterize the used FRET method. The effective donor-acceptor distance is easily retrieved either through time-resolved or steady-state fluorescence measurements. Any global fit can now be performed in seconds and minimizes the sum-of-square difference between the experimental values of the effective distance and the values obtained from the HSJE. In summary, the HSJE can give a decisive advantage in applying the speed and sensitivity of FRET spectroscopy to standing questions of polymer structure and dynamics.
Keywords: FRET; diffusion coefficient; distance distribution; fluorescence; peptide and polymer structure and dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Diffusion-enhanced Förster resonance energy transfer and the effects of external quenchers and the donor quantum yield.J Phys Chem B. 2013 Jan 10;117(1):185-98. doi: 10.1021/jp310381f. Epub 2012 Dec 31. J Phys Chem B. 2013. PMID: 23215358
-
Two Orders of Magnitude Variation of Diffusion-Enhanced Förster Resonance Energy Transfer in Polypeptide Chains.Polymers (Basel). 2018 Sep 29;10(10):1079. doi: 10.3390/polym10101079. Polymers (Basel). 2018. PMID: 30961004 Free PMC article.
-
Effect of Diffusion on Resonance Energy Transfer Rate Distributions: Implications for Distance Measurements.J Phys Chem B. 2015 Oct 1;119(39):12603-22. doi: 10.1021/acs.jpcb.5b06567. Epub 2015 Sep 21. J Phys Chem B. 2015. PMID: 26358033 Free PMC article.
-
Distance and orientation dependence of excitation energy transfer: from molecular systems to metal nanoparticles.J Phys Chem B. 2009 Feb 19;113(7):1817-32. doi: 10.1021/jp806536w. J Phys Chem B. 2009. PMID: 19128043 Review.
-
Fluorescence resonance energy transfer (FRET) and competing processes in donor-acceptor substituted DNA strands: a comparative study of ensemble and single-molecule data.J Biotechnol. 2002 Jan;82(3):211-31. doi: 10.1016/s1389-0352(01)00039-3. J Biotechnol. 2002. PMID: 11999691 Review.
Cited by
-
Internal Dynamics of Pyrene-Labeled Polyols Studied Through the Lens of Pyrene Excimer Formation.Polymers (Basel). 2025 Jul 18;17(14):1979. doi: 10.3390/polym17141979. Polymers (Basel). 2025. PMID: 40732856 Free PMC article.
-
Flexibility of Poly(alkyl methacrylate)s Characterized by Their Persistence Length Determined through Pyrene Excimer Formation.Polymers (Basel). 2024 Jul 26;16(15):2126. doi: 10.3390/polym16152126. Polymers (Basel). 2024. PMID: 39125153 Free PMC article.
References
-
- Förster T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948;2:55–75. doi: 10.1002/andp.19484370105. - DOI
-
- Haas E., Katchalski-Katzir E., Steinberg I.Z. Brownian Motion of the Ends of Oligopeptides Chains in Solution as Estimated by Energy Transfer between the Chain Ends. Biopolymers. 1978;17:11–31. doi: 10.1002/bip.1978.360170103. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

