Treatment escalation and de-escalation decisions in Crohn's disease: Delphi consensus recommendations from Japan, 2021
- PMID: 36773075
- PMCID: PMC10050046
- DOI: 10.1007/s00535-023-01958-z
Treatment escalation and de-escalation decisions in Crohn's disease: Delphi consensus recommendations from Japan, 2021
Abstract
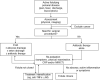
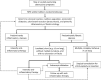
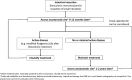
Background: We aimed to develop criteria for treatment intensification in patients with (1) luminal Crohn's disease (CD), (2) CD with perianal disease and/or fistula, (3) CD with small bowel stenosis, (4) in the postoperative setting, and (5) for discontinuing or reducing the dose of treatment in patients with CD.
Methods: PubMed and Embase were searched for studies published since 1998 which may be relevant to the five defined topics. Results were assessed for relevant studies, with preference given to data from randomized, controlled studies. For each question, a core panel of 12 gastroenterologists defined the treatment target and developed statements, based on the literature, current guidelines, and relevant additional studies. The evidence supporting each statement was graded using the Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). A modified Delphi process was used to refine statements and gain agreement from 54 Japanese specialists at in-person and online meetings conducted between October 2020 and April 2021.
Results: Seventeen statements were developed for treatment intensification in luminal CD (targeting endoscopic remission), six statements for treatment intensification in perianal/fistulizing CD (targeting healing of perianal lesions and complete closure of the fistula), six statements for treatment intensification in CD with small bowel stenosis (targeting resolution of obstructive symptoms), seven statements for treatment intensification after surgery (targeting endoscopic remission), and five statements for discontinuing or reducing the dose of treatment in patients with CD.
Conclusions: These statements provide guidance on how and when to intensify or de-intensify treatment for a broad spectrum of patients with CD.
Keywords: Consensus; Crohn’s disease; Luminal disease; Treatment escalation/de-escalation.
© 2023. The Author(s).
Conflict of interest statement
All authors received honoraria and administration costs from AbbVie to attend the meetings. Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. Hiroshi Nakase has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Mylan EPD G.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd.; research grants from AbbVie GK, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., PENTAX Medical, AYUMI Pharmaceutical Corporation; scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Kayaku Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. Motohiro Esaki has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Mitsubishi Tanabe Pharma Corporation, AbbVie GK, Janssen Pharmaceutical K.K., EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co., Ltd., AbbVie GK, Takeda Pharmaceutical Co., Ltd. Fumihito Hirai has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd.; research grants from Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., and AbbVie GK; scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation. Taku Kobayashi has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc.; research grants from AbbVie GK, Activaid, Alfresa Pharma Corporation, JMDC, Gilead Sciences, Nippon Kayaku Co., Ltd., Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Google Asia Pacific Pty. Ltd.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd.; and was an endowed chair for Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., JIMRO Co., Ltd., AbbVie GK, Zeria Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., and Mochida Pharmaceutical Co., Ltd. Katsuyoshi Matsuoka has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., AbbVie GK, EA Pharma Co., Ltd., Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd.; research grants from Janssen Pharmaceutical K.K.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd. Minoru Matsuura has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Janssen Pharmaceutical K.K. Makoto Naganuma has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for Takeda Pharmaceutical Co., Ltd., AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, JIMRO Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Pfizer Japan Inc.; research grants from Mochida Pharmaceutical Co., Ltd.; scholarship grants from AbbVie GK, Mitsubishi Tanabe Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd. Masayuki Saruta has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Ltd., Takeda Pharmaceutical Co., Ltd., EA Pharma Co., Ltd.; fees for writing a pamphlet for EA Pharma Co., Ltd.; research grants from EPS Corporation; scholarship grants from Mochida Pharmaceutical Co., Ltd., Kiichiro Tsuchiya and Motoi Uchino have no conflicts of interest to declare. Kenji Watanabe has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for AbbVie GK, EA Pharma Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., research grants from EA Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., EP-CRSU Co., Ltd., scholarship grants from AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, JIMRO Co., Ltd., Nippon Kayaku Co., Ltd., and has been an endowed chair for AbbVie GK, EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Otsuka Pharmaceutical Factory, Inc., Asahi Kasei Medical Co., Ltd., Mochida Pharmaceutical Co., Ltd. Tadakazu Hisamatsu has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for EA Pharma Co. Ltd., AbbVie GK, Celgene K.K., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Nichi-Iko Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co. Ltd., Kyorin Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc.; research grants from Alfresa Pharma Corporation, EA Pharma Co., Ltd.; scholarship grants from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co., Ltd., AbbVie GK, JIMRO Co. Ltd., Zeria Pharmaceutical Co., Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd. AbbVie, the sponsor, had no involvement in the development of the statements or the drafting of the current manuscript.
Figures
References
-
- Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145:1459–1463. doi: 10.1053/j.gastro.2013.10.047. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

