Outcomes in participants with ventilated nosocomial pneumonia and organ failure treated with ceftolozane/tazobactam versus meropenem: a subset analysis of the phase 3, randomized, controlled ASPECT-NP trial
- PMID: 36773112
- PMCID: PMC9922343
- DOI: 10.1186/s13613-022-01084-8
Outcomes in participants with ventilated nosocomial pneumonia and organ failure treated with ceftolozane/tazobactam versus meropenem: a subset analysis of the phase 3, randomized, controlled ASPECT-NP trial
Abstract
Background: The pivotal ASPECT-NP trial showed ceftolozane/tazobactam was non-inferior to meropenem for the treatment of ventilated hospital-acquired/ventilator-associated bacterial pneumonia (vHABP/VABP). Here, we evaluated treatment outcomes by degree of respiratory or cardiovascular dysfunction.
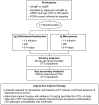
Methods: This was a subset analysis of data from ASPECT-NP, a randomized, double-blind, non-inferiority trial (ClinicalTrials.gov NCT02070757). Adults with vHABP/VABP were randomized 1:1 to 3 g ceftolozane/tazobactam or 1 g meropenem every 8 h for 8-14 days. Outcomes in participants with a baseline respiratory component of the Sequential Organ Failure Assessment (SOFA) score (R-SOFA) ≥ 2 (indicative of severe respiratory failure), cardiovascular component of the SOFA score (CV-SOFA) ≥ 2 (indicative of shock), or R-SOFA ≥ 2 plus CV-SOFA ≥ 2 were compared by treatment arm. The efficacy endpoint of primary interest was 28-day all-cause mortality. Clinical response, time to death, and microbiologic response were also evaluated.
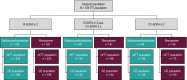
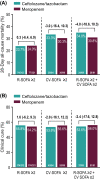
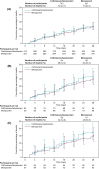
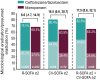
Results: There were 726 participants in the intention-to-treat population; 633 with R-SOFA ≥ 2 (312 ceftolozane/tazobactam, 321 meropenem), 183 with CV-SOFA ≥ 2 (84 ceftolozane/tazobactam, 99 meropenem), and 160 with R-SOFA ≥ 2 plus CV-SOFA ≥ 2 (69 ceftolozane/tazobactam, 91 meropenem). Baseline characteristics, including causative pathogens, were generally similar in participants with R-SOFA ≥ 2 or CV-SOFA ≥ 2 across treatment arms. The 28-day all-cause mortality rate was 23.7% and 24.0% [difference: 0.3%, 95% confidence interval (CI) - 6.4, 6.9] for R-SOFA ≥ 2, 33.3% and 30.3% (difference: - 3.0%, 95% CI - 16.4, 10.3) for CV-SOFA ≥ 2, and 34.8% and 30.8% (difference: - 4.0%, 95% CI - 18.6, 10.3), respectively, for R-SOFA ≥ 2 plus CV-SOFA ≥ 2. Clinical cure rates were as follows: 55.8% and 54.2% (difference: 1.6%, 95% CI - 6.2, 9.3) for R-SOFA ≥ 2, 53.6% and 55.6% (difference: - 2.0%, 95% CI - 16.1, 12.2) for CV-SOFA ≥ 2, and 53.6% and 56.0% (difference: - 2.4%, 95% CI - 17.6, 12.8), respectively, for R-SOFA ≥ 2 plus CV-SOFA ≥ 2. Time to death was comparable in all SOFA groups across both treatment arms. A higher rate of microbiologic eradication/presumed eradication was observed for CV-SOFA ≥ 2 and R-SOFA ≥ 2 plus CV-SOFA ≥ 2 with ceftolozane/tazobactam compared to meropenem.
Conclusions: The presence of severe respiratory failure or shock did not affect the relative efficacy of ceftolozane/tazobactam versus meropenem; either agent may be used to treat critically ill patients with vHABP/VABP.
Trial registration: ClinicalTrials.gov NCT02070757. Registered 25 February 2014, https://clinicaltrials.gov/ct2/show/NCT02070757.
Keywords: ASPECT-NP; Gram-negative; Nosocomial; Pneumonia; Pseudomonas; SOFA; Shock.
© 2023. Merck & Co., Inc., Rahway, NJ, USA and its affiliates 2023.
Conflict of interest statement
IM-L has received consulting fees and honoraria from MSD and Pfizer. AFS has received medical writing support, consulting fees, and honoraria from MSD; consulting fees from Pfizer and Shionogi; honoraria from Pfizer, Shionogi, and La Jolla. RGW is a consultant to, has received medical writing support from, and has participated in advisory committees for MSD; has received an investigator-initiated grant from Calcimedica; has participated in advisory committees for Shionogi and La Jolla; has received honoraria from BioMerieux for industry workshops at ATS, APSR, and SCCM; and has participated in the Clinical Evaluation Committee for Pfizer. MHK is supported by Barnes-Jewish Hospital Foundation and has received consulting fees and honoraria from MSD, Pfizer, and Shionogi. J-FT participated on an advisory board for MSD, Pfizer, and Shionogi on treatment of HAP/VAP; has received research grants, consulting fees, honoraria, and travel support from MSD; was past chairman of the ESCMID Study Group for Infections in Critically Ill Patients—ESGCIP; and is the principal investigator of BICCS (national research program (RCT BICCS PHRC 18-0316) on the benefit of Continuous infusion and combination therapy on the treatment of severe Gram-negative infections, including pneumonia. JAH, EJ, and CJB, and BY are current or former employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ USA.
Figures
References
-
- Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46):1800516. doi: 10.2807/1560-7917.ES.2018.23.46.1800516. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

