Use of cidofovir in a patient with severe mpox and uncontrolled HIV infection
- PMID: 36773621
- PMCID: PMC9908088
- DOI: 10.1016/S1473-3099(23)00044-0
Use of cidofovir in a patient with severe mpox and uncontrolled HIV infection
Abstract
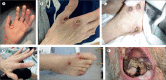
A 48-year-old man with poorly controlled HIV presented with severe human monkeypox virus (hMPXV) infection, having completed 2 weeks of tecovirimat at another hospital. He had painful, ulcerating skin lesions on most of his body and oropharyngeal cavity, with subsequent Ludwig's angina requiring repeated surgical interventions. Despite commencing a second, prolonged course of tecovirimat, he did not objectively improve, and new lesions were still noted at day 24. Discussion at the UK National Health Service England High Consequence Infectious Diseases Network recommended the use of 3% topical and then intravenous cidofovir, which was given at 5 mg/kg; the patient made a noticeable improvement after the first intravenous dose. He received further intravenous doses at 7 days and 21 days after the dose and was discharged at day 52. Cidofovir is not licensed for use in treatment of hMPXV infection. Data for cidofovir use in hMPXV are restricted to studies in animals. Four other documented cases of cidofovir use against hMPXV have been reported in the USA in 2022, but we present its first use in the UK. The scarcity of studies into the use of cidofovir in this condition clearly shows the need for robust studies to assess efficacy, optimum dosage, timing, and route of administration.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DA-J has received consultancy fees or honoraria from Gilead and Pulmocide and has stock or stock options in Pulmocide. MG has received consultancy fees from Pfizer. FD receives a Clinical Academic Research Partnerships Fellowship from the Medical Research Council. All other authors declare no competing interests.
Figures

References
-
- WHO WHO recommends new name for monkeypox disease. 2022. https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-mon...
-
- WHO Multi-country monkeypox outbreak: situation update 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390
-
- WHO Director-General declares the ongoing monkeypox outbreak a public health emergency of international concern. 2022. https://www.who.int/europe/news/item/23-07-2022-who-director-general-dec... - PMC - PubMed
-
- Centers for Disease Control and Prevention 2022 monkeypox outbreak global map. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

