Cost-Effectiveness Analysis of the South African Infant National Immunization Program for the Prevention of Pneumococcal Disease
- PMID: 36774428
- PMCID: PMC9922201
- DOI: 10.1007/s40121-023-00767-4
Cost-Effectiveness Analysis of the South African Infant National Immunization Program for the Prevention of Pneumococcal Disease
Abstract
Introduction: Pneumococcal disease, which presents a substantial health and economic burden, is prevented through pneumococcal vaccination programs. We assessed the impact of switching from a 13-valent-based (PCV13) to lower 10-valent-based (PCV10-GlaxoSmithKline [GSK] or PCV10-Serum Institute of India [SII]) or higher-valent (PCV15 or PCV20) vaccination programs in South Africa.
Methods: A previously published decision-analytic model was adapted to a South African setting. Historical invasive pneumococcal disease (IPD) incidence data were used to project IPD incidence over time for each vaccination program on the basis of serotype coverage. Historical incidence (IPD, pneumonia, otitis media), mortality, costs, and utilities were obtained from the published literature. Cases of disease, direct medical costs (i.e., vaccination, IPD, pneumonia, and otitis media costs) (in 2022 South African rands), life-years, quality-adjusted life-years (QALY), and incremental cost per QALY were estimated over a 5- and 10-year horizon for PCV13 and the PCV10 vaccines. Additionally, a public health impact analysis was conducted comparing PCV13, PCV15, and PCV20.
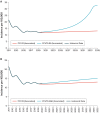
Results: Continuing use of PCV13 would substantially reduce disease incidence over time compared with switching to either of the PCV10 lower-valent vaccines. Cases of IPD were reduced by 4.22% and 34.70% when PCV13 was compared to PCV10-GSK and PCV10-SII, respectively. PCV13 was also found to be cost saving over 5- and 10-year time horizons compared with PCV10-SII and to be cost-effective over a 5-year time horizon and cost-saving over a 10-year time horizon compared with PCV10-GSK. PCV20 was consistently estimated to prevent more cases than the PCV10 vaccines, PCV13, or PCV15.
Conclusions: Switching from a higher-valent to a lower-valent vaccine may lead to disease incidence re-emergence caused by previously covered serotypes. Maintaining PCV13 was estimated to improve public health further by averting additional pneumococcal disease cases and saving more lives and also to reduce total costs in most scenarios. Higher-valent PCVs can achieve the greatest public health impact in the pediatric vaccination program in South Africa.
Keywords: PCV; PCV13; PCV20; Pneumococcal disease; Vaccine.
© 2023. Pfizer Inc.
Figures


Similar articles
-
Cost-Effectiveness Analysis of Pneumococcal Vaccines in the Pediatric Population: A Systematic Review.Healthcare (Basel). 2024 Sep 29;12(19):1950. doi: 10.3390/healthcare12191950. Healthcare (Basel). 2024. PMID: 39408130 Free PMC article. Review.
-
Cost-effectiveness of PCV20 to Prevent Pneumococcal Disease in the Pediatric Population: A German Societal Perspective Analysis.Infect Dis Ther. 2024 Jun;13(6):1333-1358. doi: 10.1007/s40121-024-00977-4. Epub 2024 May 11. Infect Dis Ther. 2024. PMID: 38733494 Free PMC article.
-
Cost-Effectiveness of 20-Valent Pneumococcal Conjugate Vaccine in Argentinean Adults.Infect Dis Ther. 2024 Jun;13(6):1235-1251. doi: 10.1007/s40121-024-00972-9. Epub 2024 May 3. Infect Dis Ther. 2024. PMID: 38700655 Free PMC article.
-
Clinical and Economic Impact of a Potential Switch from 13-Valent to 10-Valent Pneumococcal Conjugate Infant Vaccination in Canada.Infect Dis Ther. 2018 Sep;7(3):353-371. doi: 10.1007/s40121-018-0206-1. Epub 2018 Jun 22. Infect Dis Ther. 2018. PMID: 29934878 Free PMC article.
-
The impact of routine childhood immunization with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: a systematic literature review.Expert Rev Vaccines. 2019 Oct;18(10):1069-1089. doi: 10.1080/14760584.2019.1676155. Epub 2019 Oct 22. Expert Rev Vaccines. 2019. PMID: 31585049
Cited by
-
Estimating the Clinical and Economic Impact of Switching from the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) to Higher-Valent Options in Greek Infants.Vaccines (Basel). 2023 Aug 15;11(8):1369. doi: 10.3390/vaccines11081369. Vaccines (Basel). 2023. PMID: 37631937 Free PMC article.
-
Nasopharyngeal carriage of Streptococcus pneumoniae among children and their household members in southern Mozambique five years after PCV10 introduction.Vaccine. 2025 Feb 15;47:126691. doi: 10.1016/j.vaccine.2024.126691. Epub 2025 Jan 8. Vaccine. 2025. PMID: 39787794 Free PMC article.
-
Cost-Effectiveness Analysis of Pneumococcal Vaccines in the Pediatric Population: A Systematic Review.Healthcare (Basel). 2024 Sep 29;12(19):1950. doi: 10.3390/healthcare12191950. Healthcare (Basel). 2024. PMID: 39408130 Free PMC article. Review.
-
Delayed Transition to 20-Valent Pneumococcal Conjugate Vaccine in Pediatric National Immunization Programs: Forgone Public Health and Economic Benefit.Infect Dis Ther. 2025 Mar;14(3):501-525. doi: 10.1007/s40121-025-01108-3. Epub 2025 Feb 3. Infect Dis Ther. 2025. PMID: 39899200 Free PMC article.
-
A Novel Approach to Estimate the Impact of PCV20 Immunization in Children by Incorporating Indirect Effects to Generate the Number Needed to Vaccinate.Vaccines (Basel). 2025 Jul 29;13(8):805. doi: 10.3390/vaccines13080805. Vaccines (Basel). 2025. PMID: 40872892 Free PMC article.
References
-
- World Health Organization. Pneumococcal disease: the leading vaccine-preventable cause of death in children under five. 16 November 2016. https://www.gavi.org/pneumococcal-disease-leading-vaccine-preventable-ca.... Accessed 24 August 2021.
-
- Yun J, Kleynhans J, Moyes J, et al. Epidemiology of respiratory pathogens from the influenza-like illness and pneumonia surveillance programmes, South Africa. 2019. https://www.nicd.ac.za/wp-content/uploads/2020/10/EPIDEMIOLOGY-OF-RESPIR.... Accessed 17 August 2022.
LinkOut - more resources
Full Text Sources

