HLA-haploidentical hematopoietic stem cells transplantation with regulatory and conventional T-cell adoptive immunotherapy in pediatric patients with very high-risk acute leukemia
- PMID: 36774432
- PMCID: PMC9919737
- DOI: 10.1038/s41409-023-01911-x
HLA-haploidentical hematopoietic stem cells transplantation with regulatory and conventional T-cell adoptive immunotherapy in pediatric patients with very high-risk acute leukemia
Abstract
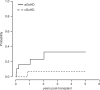
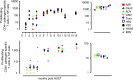
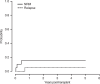
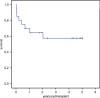
Allogeneic hematopoietic stem cell transplantation (HSCT) is still needed for many children with very high-risk acute leukemia. An HLA-haploidentical family donor is a suitable option for those without an HLA-matched donor. Here we present outcomes of a novel HLA-haploidentical HSCT (haplo-HSCT) strategy with adoptive immunotherapy with thymic-derived CD4+CD25+ FoxP3+ regulatory T cells (Tregs) and conventional T cells (Tcons) performed between January 2017 and July 2021 in 20 children with high-risk leukemia. Median age was 14.5 years (range, 4-21), 15 had acute lymphoblastic leukemia, 5 acute myeloid leukemia. The conditioning regimen included total body irradiation (TBI), thiotepa, fludarabine, cyclophosphamide. Grafts contained a megadose of CD34+ cells (mean 12.4 × 106/Kg), Tregs (2 × 106/Kg) and Tcons (0.5-1 × 106/Kg). All patients achieved primary, sustained full-donor engraftment. Only one patient relapsed (5%). The incidence of non-relapse mortality was 15% (3/20 patients). Five/20 patients developed ≥ grade 2 acute Graft versus Host Disease (aGvHD). It resolved in 4 who are alive and disease-free; 1 patient developed chronic GvHD (cGvHD). The probability of GRFS was 60 ± 0.5% (95% CI: 2.1-4.2) (Fig. 6), CRFS was 79 ± 0.9% (95% CI: 3.2-4.9) as 16/20 patients are alive and leukemia-free. The median follow-up was 2.1 years (range 0.5 months-5.1 years). This innovative approach was associated with very promising outcomes of HSCT strategy in pediatric patients.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477–85. doi: 10.1182/blood-2008-01-132837. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

