Early computational detection of potential high-risk SARS-CoV-2 variants
- PMID: 36774893
- PMCID: PMC9892295
- DOI: 10.1016/j.compbiomed.2023.106618
Early computational detection of potential high-risk SARS-CoV-2 variants
Abstract
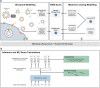
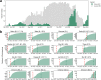
The ongoing COVID-19 pandemic is leading to the discovery of hundreds of novel SARS-CoV-2 variants daily. While most variants do not impact the course of the pandemic, some variants pose an increased risk when the acquired mutations allow better evasion of antibody neutralisation or increased transmissibility. Early detection of such high-risk variants (HRVs) is paramount for the proper management of the pandemic. However, experimental assays to determine immune evasion and transmissibility characteristics of new variants are resource-intensive and time-consuming, potentially leading to delays in appropriate responses by decision makers. Presented herein is a novel in silico approach combining spike (S) protein structure modelling and large protein transformer language models on S protein sequences to accurately rank SARS-CoV-2 variants for immune escape and fitness potential. Both metrics were experimentally validated using in vitro pseudovirus-based neutralisation test and binding assays and were subsequently combined to explore the changing landscape of the pandemic and to create an automated Early Warning System (EWS) capable of evaluating new variants in minutes and risk-monitoring variant lineages in near real-time. The system accurately pinpoints the putatively dangerous variants by selecting on average less than 0.3% of the novel variants each week. The EWS flagged all 16 variants designated by the World Health Organization (WHO) as variants of interest (VOIs) if applicable or variants of concern (VOCs) otherwise with an average lead time of more than one and a half months ahead of their designation as such.
Copyright © 2023 BioNTech and InstaDeep. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest U.S. is a management board member and employee at BioNTech SE. A.M., B.G.L. and B.S. are employees at BioNTech SE. A.P. and Y.L. are employees at BioNTech US: U.S., A.M., Y.L., and A.P. are inventors on patents and patent applications related to RNA technology and/or the COVID-19 vaccine. U.S., A.M., B.G.L., and B.S. have securities from BioNTech SE. K.B. is a management board member and employee at InstaDeep Ltd. M.J.S., Y.F., T.P., N.L.C., A.L., I.K., A.K. and A.U.L. are employees of InstaDeep Ltd or its subsidiaries. K.B., M.J.S., Y.F., T.P., N.L.C., and A.L. are inventors of patents and patent applications related to machine learning technology. K.B., M.J.S., Y.F., T.P., N.L.C., and A.L. have securities from InstaDeep Ltd.
Figures




References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

