Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial
- PMID: 36774933
- PMCID: PMC10259621
- DOI: 10.1016/S0140-6736(22)02574-0
Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial
Abstract
Background: Effective adjuvant therapy for patients with resected localised renal cell carcinoma represents an unmet need, with surveillance being the standard of care. We report results from part A of a phase 3, randomised trial that aimed to assess the efficacy and safety of adjuvant nivolumab plus ipilimumab versus placebo.
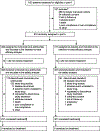
Methods: The double-blind, randomised, phase 3 CheckMate 914 trial enrolled patients with localised clear cell renal cell carcinoma who were at high risk of relapse after radical or partial nephrectomy between 4-12 weeks before random assignment. Part A, reported herein, was done in 145 hospitals and cancer centres across 20 countries. Patients were randomly assigned (1:1) to nivolumab (240 mg) intravenously every 2 weeks for 12 doses plus ipilimumab (1 mg/kg) intravenously every 6 weeks for four doses, or matching placebo, via an interactive response technology system. The expected treatment period was 24 weeks, and treatment could be continued until week 36, allowing for treatment delays. Randomisation was stratified by TNM stage and nephrectomy (partial vs radical). The primary endpoint was disease-free survival according to masked independent central review; safety was a secondary endpoint. Disease-free survival was analysed in all randomly assigned patients (intention-to-treat population); exposure, safety, and tolerability were analysed in all patients who received at least one dose of study drug (all-treated population). This study is registered with ClinicalTrials.gov, NCT03138512.
Findings: Between Aug 28, 2017, and March 16, 2021, 816 patients were randomly assigned to receive either adjuvant nivolumab plus ipilimumab (405 patients) or placebo (411 patients). 580 (71%) of 816 patients were male and 236 (29%) patients were female. With a median follow-up of 37·0 months (IQR 31·3-43·7), median disease-free survival was not reached in the nivolumab plus ipilimumab group and was 50·7 months (95% CI 48·1 to not estimable) in the placebo group (hazard ratio 0·92, 95% CI 0·71-1·19; p=0·53). The number of events required for the planned overall survival interim analysis was not reached at the time of the data cutoff, and only 61 events occurred (33 in the nivolumab plus ipilimumab group and 28 in the placebo group). 155 (38%) of 404 patients who received nivolumab plus ipilimumab and 42 (10%) of 407 patients who received placebo had grade 3-5 adverse events. All-cause adverse events of any grade led to discontinuation of nivolumab plus ipilimumab in 129 (32%) of 404 treated patients and of placebo in nine (2%) of 407 treated patients. Four deaths were attributed to treatment with nivolumab plus ipilimumab and no deaths were attributed to treatment with placebo.
Interpretation: Adjuvant therapy with nivolumab plus ipilimumab did not improve disease-free survival versus placebo in patients with localised renal cell carcinoma at high risk of recurrence after nephrectomy. Our study results do not support this regimen for the adjuvant treatment of renal cell carcinoma.
Funding: Bristol Myers Squibb and Ono Pharmaceutical.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests RJM reports clinical trial support (institutional) from Bristol Myers Squibb (BMS) for this manuscript; advisory board fees from AstraZeneca, AVEO, Eisai, EMD Serono, Exelixis, Genentech/Roche, Incyte, Lilly Oncology, Merck, Novartis, and Pfizer; and fees (institutional) for coordinating principal investigator from AVEO, BMS, Eisai, Exelixis, Genentech/Roche, Merck, and Pfizer. VG reports medical writing and processing charges from BMS for this manuscript, research grants (institutional) from BMS, Ipsen, MSD, and Pfizer; speakers' bureau fees from Astellas, AstraZeneca, BMS, Eisai, Ipsen, Janssen-Cilag, Merck Serono, MSD, Nanobiotix, Novartis, Ono Pharmaceutical, Pfizer, and Roche; advisory board fees from Apogepha, BMS, Debiopharm, Eisai, EUSA, MSD, Nanobiotix, Oncorena, PCI Biotech, Pfizer, Roche, and Merck Serono; leadership roles (unpaid) with AIO and Das Lebenshaus for patient advocacy; stock options from AstraZeneca, BMS, MSD, and Seattle Genetics; steering committee membership for BMS, Eisai, Ipsen, and Novartis; and being trial chair for PharmaMar. YT reports research grants from Chugai and Ono Pharmaceutical; speakers' bureau fees from Astellas, BMS, Merck, and Ono Pharmaceutical; and advisory board fees from Eisai, Ono Pharmaceutical, and MSD. SB reports research grants from Novartis; speakers' bureau fees from BMS, Ipsen, MSD, and Novartis; payment for expert testimony from MSD, BMS, Ipsen, and Pfizer; advisory board fees from BMS, Ipsen, MSD, Novartis, and Pfizer; fees (institutional) for being a coordinating principal investigator from BMS and Ipsen; and being a member of AIOM and Meet-URO group. PB reports research grants from BMS, Ipsen, MSD, Pfizer, Merck, AstraZeneca, and Janssen-Cilag; consulting fees from BMS, Ipsen, MSD, Merck, Pfizer, Janssen-Cilag, Astellas, Amgen, and Gilead; honoraria from BMS, Ipsen, MSD, Merck, Pfizer, Janssen-Cilag, Astellas, Seagen, Novartis, and AAA; travel expense support from Pfizer, Merck, BMS, and Ipsen; and advisory board fees from Merck and Pfizer. JCG reports medical writing support from BMS for this manuscript; research grants (institutional) from BeiGene; honoraria from MSD and GlaxoSmithKline; travel expense support from AstraZeneca; advisory board fees from BMS, GlaxoSmithKline, MSD, Eisai, Janssen, and AstraZeneca; stock options from ICON Cancer Centres; meeting chair fees from Ipsen; and local principal investigator fees from BMS. J-BL reports consulting fees from BMS, Merck, Sanofi, Paladin, Pfizer, Novartis, Knights Pharmacy, Verity, and AbbVie; speakers' bureau fees from Tersera and Tolmar; advisory board fees from BMS, Knights Pharmacy, and Verity; being a local principal investigator for BMS; and being a member of AUA, Canadian Uro-Oncology Group, and Canadian Urological Association. LA reports research grants (institutional) from BMS, consulting fees (institutional) from BMS, Ipsen, Roche, Novartis, Pfizer, Astellas Pharma, Merck, MSD, AstraZeneca, Janssen, and Eisai; and travel support from BMS and MSD. SG reports advisory board fees from AVEO, Bayer, BMS, Corvus, Eisai, EMD Serono, Exelixis, Merck, Pfizer, QED Therapeutics, Sanofi/Genzyme, and Seattle Genetics; and non-financial interests from Agensys, Aravive, AVEO, Bayer, BMS, Calithera, Corvus, Eisai, Exelixis, Gilead, Merck, Novartis, Pfizer, Seattle Genetics, and Surface Oncology. BSh reports research grants (institutional) from Allogene and Rebiotix; royalties from Up to Date; consulting fees from Veracyte and Merck; speakers' bureau fees from Merck; advisory board fees from Genentech, Merck, and Johnson & Johnson; and board leadership fees from the National Cancer Institute PDQ. JSo reports consulting fees from Apexigen, Jazz Pharmaceuticals, and Iovance Biotherapeutics; and honoraria from Apexigen, Jazz Pharmaceuticals, and Iovance Biotherapeutics. MS reports support from BMS for this manuscript; grants from Pfizer, GlaxoSmithKline, AVEO, BMS, Novartis, Bayer, Roche/Genentech, Immatics, Wilex, Ipsen, Exelixis, and Eisai; consulting fees from Pfizer, GlaxoSmithKline, Novartis, Bayer, Roche AVEO, EUSA Pharma, Astellas, Ipsen, Exelixis, Peloton Therapeutics, Eisai, BMS, MSD, and Apogepha; and travel support from Pfizer, EUSA Pharma, Ipsen, Eisai, BMS, MSD, and Apogepha. BSi, JSp, and AC are employed by and have stock ownership in BMS. AB reports research grants from Pfizer, advisory board fees from the International Kidney Cancer Coalition and the Kidney Cancer Association; and being a local principal investigator and steering committee member for BMS and Roche/Genentech. All other authors declare no competing interests.
Figures
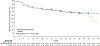

Comment in
-
Adjuvant ipilimumab and nivolumab in renal cell carcinoma: more questions than answers.Lancet. 2023 Mar 11;401(10379):796-798. doi: 10.1016/S0140-6736(22)02631-9. Epub 2023 Feb 9. Lancet. 2023. PMID: 36774931 No abstract available.
References
-
- Martinez Chanza N, Tripathi A, Harshman LC. Adjuvant therapy options in renal cell carcinoma: Where do we stand? Curr Treat Options Oncol 2019; 20: 44. - PubMed
-
- Harshman LC, Xie W, Moreira RB, et al. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: a trial-level meta-analysis. Cancer 2018; 124: 925–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

