Quantifying the effect of delaying the second COVID-19 vaccine dose in England: a mathematical modelling study
- PMID: 36774945
- PMCID: PMC9910835
- DOI: 10.1016/S2468-2667(22)00337-1
Quantifying the effect of delaying the second COVID-19 vaccine dose in England: a mathematical modelling study
Abstract
Background: The UK was the first country to start national COVID-19 vaccination programmes, initially administering doses 3 weeks apart. However, early evidence of high vaccine effectiveness after the first dose and the emergence of the SARS-CoV-2 alpha variant prompted the UK to extend the interval between doses to 12 weeks. In this study, we aimed to quantify the effect of delaying the second vaccine dose in England.
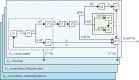
Methods: We used a previously described model of SARS-CoV-2 transmission, calibrated to COVID-19 surveillance data from England, including hospital admissions, hospital occupancy, seroprevalence data, and population-level PCR testing data, using a Bayesian evidence-synthesis framework. We modelled and compared the epidemic trajectory in the counterfactual scenario in which vaccine doses were administered 3 weeks apart against the real reported vaccine roll-out schedule of 12 weeks. We estimated and compared the resulting numbers of daily infections, hospital admissions, and deaths. In sensitivity analyses, we investigated scenarios spanning a range of vaccine effectiveness and waning assumptions.
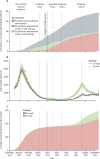
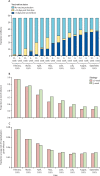
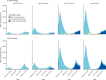
Findings: In the period from Dec 8, 2020, to Sept 13, 2021, the number of individuals who received a first vaccine dose was higher under the 12-week strategy than the 3-week strategy. For this period, we estimated that delaying the interval between the first and second COVID-19 vaccine doses from 3 to 12 weeks averted a median (calculated as the median of the posterior sample) of 58 000 COVID-19 hospital admissions (291 000 cumulative hospitalisations [95% credible interval 275 000-319 000] under the 3-week strategy vs 233 000 [229 000-238 000] under the 12-week strategy) and 10 100 deaths (64 800 deaths [60 200-68 900] vs 54 700 [52 800-55 600]). Similarly, we estimated that the 3-week strategy would have resulted in more infections compared with the 12-week strategy. Across all sensitivity analyses the 3-week strategy resulted in a greater number of hospital admissions. In results by age group, the 12-week strategy led to more hospitalisations and deaths in older people in spring 2021, but fewer following the emergence of the delta variant during summer 2021.
Interpretation: England's delayed-second-dose vaccination strategy was informed by early real-world data on vaccine effectiveness in the context of limited vaccine supplies in a growing epidemic. Our study shows that rapidly providing partial (single-dose) vaccine-induced protection to a larger proportion of the population was successful in reducing the burden of COVID-19 hospitalisations and deaths overall.
Funding: UK National Institute for Health Research; UK Medical Research Council; Community Jameel; Wellcome Trust; UK Foreign, Commonwealth and Development Office; Australian National Health and Medical Research Council; and EU.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AC has received payment from Pfizer for teaching of mathematical modelling of infectious diseases. KAMG has received honoraria from Wellcome Genome Campus for lectures. LKW has received consultancy payments from the Wellcome Trust. ABH has received consultancy payments from WHO for COVID-19-related work; provides advice on COVID-19 modelling to the New South Wales Ministry of Health, Australia; and was previously engaged by Pfizer to advise on modelling of RSV vaccination strategies, for which she received no financial compensation. RS and NI are currently employed by the Wellcome Trust. All other authors declare no competing interests.
Figures

References
-
- Medicines and Healthcare products Regulatory Agency Regulatory approval of Pfizer/BioNTech vaccine for COVID-19. Dec 2, 2020. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer...
-
- Medicines and Healthcare products Regulatory Agency Regulatory approval of COVID-19 vaccine AstraZeneca. Dec 30, 2020. https://www.gov.uk/government/publications/regulatory-approval-of-covid-...
-
- Department of Health & Social Care Optimising the COVID-19 vaccination programme for maximum short-term impact. Jan 26, 2021. https://www.gov.uk/government/publications/prioritising-the-first-covid-...
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

