Neuroimmune interactions with binge alcohol drinking in the cerebellum of IL-6 transgenic mice
- PMID: 36775097
- PMCID: PMC10029700
- DOI: 10.1016/j.neuropharm.2023.109455
Neuroimmune interactions with binge alcohol drinking in the cerebellum of IL-6 transgenic mice
Abstract
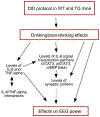
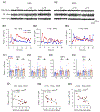
The neuroimmune system of the brain, which is comprised primarily of astrocytes and microglia, regulates a variety of homeostatic mechanisms that underlie normal brain function. Numerous conditions, including alcohol consumption, can disrupt this regulatory process by altering brain levels of neuroimmune factors. Alcohol and neuroimmune factors, such as proinflammatory cytokines IL-6 and TNF-alpha, act at similar targets in the brain, including excitatory and inhibitory synaptic transmission. Thus, alcohol-induced production of IL-6 and/or TNF-alpha could be important contributing factors to the effects of alcohol on the brain. Recent studies indicate that IL-6 plays a role in alcohol drinking and the effects of alcohol on the brain activity following the cessation of alcohol consumption (post-alcohol period), however information on these topics is limited. Here we used homozygous and heterozygous female and male transgenic mice with increased astrocyte expression of IL-6 to examined further the interactions between alcohol and IL-6 with respect to voluntary alcohol drinking, brain activity during the post-alcohol period, IL-6 signal transduction, and expression of synaptic proteins. Wildtype littermates (WT) served as controls. The transgenic mice model brain neuroimmune status with respect to IL-6 in subjects with a history of persistent alcohol use. Results showed a genotype dependent reduction in voluntary alcohol consumption in the Drinking in the Dark protocol and in frequency-dependent relationships between brain activity in EEG recordings during the post-alcohol period and alcohol consumption. IL-6, TNF-alpha, IL-6 signal transduction partners pSTAT3 and c/EBP beta, and synaptic proteins were shown to play a role in these genotypic effects.
Keywords: EEG; IL-6; STAT3; Signal transduction; Synapse; TNF-alpha; c/EBP beta; pSTAT3.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflict of interest to declare.
Figures
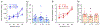



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

