Multicenter Long-Term Follow-Up of Allogeneic Hematopoietic Cell Transplantation with Omidubicel: A Pooled Analysis of Five Prospective Clinical Trials
- PMID: 36775201
- PMCID: PMC10149622
- DOI: 10.1016/j.jtct.2023.01.031
Multicenter Long-Term Follow-Up of Allogeneic Hematopoietic Cell Transplantation with Omidubicel: A Pooled Analysis of Five Prospective Clinical Trials
Erratum in
-
Corrigendum to 'Multicenter Long-Term Follow-Up of Allogeneic Hematopoietic Cell Transplantation with Omidubicel: A Pooled Analysis of Five Prospective Clinical Trials'.Transplant Cell Ther. 2024 Aug;30(8):821. doi: 10.1016/j.jtct.2023.06.009. Epub 2023 Jul 5. Transplant Cell Ther. 2024. PMID: 37421972 No abstract available.
Abstract
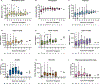
Omidubicel is an umbilical cord blood (UCB)-derived ex vivo-expanded cellular therapy product that has demonstrated faster engraftment and fewer infections compared with unmanipulated UCB in allogeneic hematopoietic cell transplantation. Although the early benefits of omidubicel have been established, long-term outcomes remain unknown. We report on a planned pooled analysis of 5 multicenter clinical trials including 105 patients with hematologic malignancies or sickle cell hemoglobinopathy who underwent omidubicel transplantation at 26 academic transplantation centers worldwide. With a median follow-up of 22 months (range, .3 to 122 months), the 3-year estimated overall survival and disease-free survival were 62.5% and 54.0%, respectively. With up to 10 years of follow-up, omidubicel showed durable trilineage hematopoiesis. Serial quantitative assessments of CD3+, CD4+, CD8+, CD19+, CD116+CD56+, and CD123+ immune subsets revealed median counts remaining within normal ranges through up to 8 years of follow-up. Secondary graft failure occurred in 5 patients (5%) in the first year, with no late cases reported. One case of donor-derived myeloid neoplasm was reported at 40 months post-transplantation. This was also observed in a control arm patient who received only unmanipulated UCB. Overall, omidubicel demonstrated stable trilineage hematopoiesis, immune competence, and graft durability in extended follow-up.
Keywords: Allogeneic stem cell transplantation; Clinical trial; Cord blood; Ex vivo expansion; Long-term follow-up.
Copyright © 2023 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Disclosures:
Authors MEH, GS, JS, and PM have received institutional research grants from Gamida Cell. RTM reports consultancy and/or advisory roles with Artiva Therapeutics, Bristol-Myers Squibb/Celgene, CRISPR Therapeutics, Incyte, Kite and Novartis, research funding from BMS and Novartis, DSMB for Athersys, Novartis, NMDP and Century Therapeutics, and patents with Athersys. The remaining authors have no disclosures.
Figures

References
-
- Peled T, Shoham H, Aschengrau D, et al.: Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol 40:342–55.e1, 2012 - PubMed

