Assessing Bulbar Function in Spinal Muscular Atrophy Using Patient-Reported Outcomes
- PMID: 36776075
- PMCID: PMC10258884
- DOI: 10.3233/JND-221573
Assessing Bulbar Function in Spinal Muscular Atrophy Using Patient-Reported Outcomes
Abstract
Background: Novel Spinal Muscular Atrophy (SMA) treatments have demonstrated improvements on motor measures that are clearly distinct from the natural history of progressive decline. Comparable measures are needed to monitor bulbar function, which is affected in severe SMA.
Objective: To assess bulbar function with patient-reported outcome measures (PROs) and determine their relationships with clinical characteristics.
Methods: We recruited 47 non-ambulatory participants (mean (SD) age = 29.8 (13.7) years, range = 10.3-73.2) with SMA. PROs including Voice Handicap Index (VHI) and Eating Assessment Tool-10 (EAT-10) were collected alongside clinical characteristics and standardized motor assessments. Associations were assessed using Spearman correlation coefficients and group comparisons were performed using Wilcoxon rank sum tests.
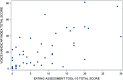
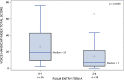
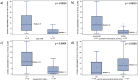
Results: A majority of the 47 participants were SMA type 2 (70.2%), non-sitters (78.7%), 3 copies of SMN2 (77.5%), and using respiratory support (66.0%). A majority (94%) reported voice issues primarily in 8/30 VHI questions. Problems included: difficulty understanding me in a noisy room (87.2%); difficult for people to hear me (74.5%); and people ask me to repeat when speaking face-to-face (72.3%). A majority (85.1%) reported swallowing issues primarily in 3/10 EAT-10 questions: swallowing pills (68.1%); food sticks to my throat (66.0%); and swallowing solids (61.7%). The two PROs were moderately associated (rs = 0.66).
Conclusions: Weaker individuals with SMA experience bulbar problems including difficulties with voice and swallowing. Further refinement and assessment of functional bulbar scales will help determine their relevance and responsiveness to changes in SMA. Additional study is needed to quantify bulbar changes caused by SMA and their response to disease-modifying treatments.
Keywords: Spinal muscular atrophy; communication; deglutition; dysphagia; patient-reported outcome measures; speech; swallow; voice.
Conflict of interest statement
S. D. Y. has been a member of advisory boards for Biogen, Roche/Genentech, and Scholar Rock; received personal compensation for activities with Biogen, Cure SMA, and Scholar Rock as a consultant; and received research support from Cure SMA and the SMA Foundation.
A. P. has received research support from the SMA Foundation; served as a consultant for Biogen; and has served on advisory boards for AveXis, Biogen, Roche, and Scholar Rock.
T. D. has been an advisory board member of Cure SMA, Biogen, Cytokinetics, Roche/Genentech, Scholar Rock, Sarepta, Bristol Myers Squibb, Audentes, and Novartis and served as a consultant for Roche, Audentes, and Biohaven; receives research support and grants from Biogen and Ionis.
K. M. has received grant funding and consulting income from Biogen and AveXis but has no financial interest in these companies. She is founder and scientific director of nuBorn Medical and Science Stand. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
S. S. has received consulting fees from Roche, Genetech, Inc., doc.ai.
E. M. has no disclosures to report.
C. D. has no disclosures to report.
W. T. has no disclosures to report.
D. P. has no disclosures to report.
A. L. has no disclosures to report.
A. R. has no disclosures to report.
C. W. has served on advisory boards and speaker bureaus for Biogen.
W. M. has no disclosures to report.
M. M. has received research support from the NIH, FDA, Cure SMA, and PTC Therapeutics; served as a consultant for Fulcrum Therapeutics, Inc. and NeuroDerm, Ltd.; and served on Data and Safety Monitoring Boards for NIH, Eli Lilly and Company, Catabasis Pharmaceuticals, Inc., Vaccinex, Inc., Neurocrine Biosciences, Inc., Voyager Therapeutics, Prilenia Therapeutics Development, Ltd., ReveraGen BioPharma, Inc., and NS Pharma, Inc
B. T. D. has been a member of advisory boards for AveXis, Biogen, Cytokinetics, Dynacure, PTC, Roche, and Sarepta; received research support from Cure SMA, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, the Slaney Family Fund for SMA, the SMA Foundation, and the Working on Walking Fund; and received grants from Biogen and Ionis Pharmaceuticals, Inc. during the ENDEAR, CHERISH, CS2, CS12, and CS11 studies, and from Cytokinetics, Fibrogen, PTC, Roche, Santhera, Sarepta, and Summit, and reports no personal financial interests in these companies.
J. W. D. has received consulting fees from Audentes, Biogen, Ionis Pharmaceuticals, Roche/Genentech Pharmaceuticals, Cytokinetics, Pfizer, AveXis, AMO Pharmaceuticals, Sarepta Therapeutics, Santhera Pharmaceuticals, and Scholar Rock. He has received grant support from Biogen; Ionis Pharmaceuticals, Cytokinetics, Roche Pharmaceuticals, AveXis, Sanofi-Genzyme, Sarepta Therapeutics, and Scholar Rock.
Figures

References
-
- Darras BT, Markowitz JA, Monani UR, et al.. Chapter 8 –Spinal Muscular Atrophies. In: Basil T. Darras Monique M. Ryan, Darryl C. De Vivo HRJ (ed) Neuromuscular Disorders of Infancy, Childhood, and Adolescence (Second Edition). San Diego: Academic Press, 117–45.
-
- Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord. 1995;5:3–5. - PubMed
-
- Mercuri E, Finkel RS, Muntoni F, et al.. Diagnosis and management of spinal muscular atrophy: Part Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28:103–15. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

