Hepatic arterial infusion chemotherapy versus sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombus: An updated meta-analysis and systematic review
- PMID: 36776344
- PMCID: PMC9911796
- DOI: 10.3389/fonc.2023.1085166
Hepatic arterial infusion chemotherapy versus sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombus: An updated meta-analysis and systematic review
Abstract
Background: Sorafenib was the first drug approved for advanced hepatocellular carcinoma (HCC). However, it is limited by poor efficacy for HCC with portal vein tumor thrombus (PVTT). Some studies suggested that hepatic artery infusion chemotherapy (HAIC) could provide survival benefits to patients with advanced HCC with PVTT.
Aim: The study aimed to compare the efficacy of HAIC versus sorafenib in patients with HCC accompanied by PVTT.
Methods: The PubMed, Embase, and Cochrane Library databases were searched for studies published until September 2022. Statistical analyses were performed using Stata SE 15 software.
Results: Eight studies with 672 patients, 403 in the HAIC group and 269 in the sorafenib group, were included in the meta-analysis. The rates of complete response (RR=3.88, 95%CI:1.35-11.16, P=0.01), partial response (RR=3.46, 95%CI:1.94-6.18, P<0.0001), objective response rate (RR=4.21, 95%CI:2.44-7.28, P<0.00001) and disease control rate (RR=1.73, 95%CI:1.28-2.35, P=0.0004) were significantly higher in the HAIC group compared to the sorafenib group, whereas the progressive disease rate (RR=0.57, 95%CI:0.40-0.80, P=0.02) was significantly lower in the former. In contrast, the stable disease rate (RR=1.10, 95%CI (0.69-1.76), P=0.68) was similar in both groups. The overall survival (HR=0.50, 95%CI:0.40-0.63, P<0.05) and progression-free survival (HR=0.49, 95%CI:0.35-0.67, P<0.05) rates were significantly higher in the HAIC group compared to the sorafenib group.
Conclusion: HAIC has better efficacy against HCC with PVTT than sorafenib and may be considered an alternative to the latter. However, more high-quality randomized control trials and longer follow-ups are needed to verify our findings.
Keywords: hepatic arterial infusion chemotherapy; hepatocellular carcinoma; meta-analysis; portal vein tumor thrombosis; prognosis; sorafenib.
Copyright © 2023 Zhang, Ouyang, Huang and Che.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
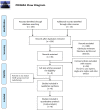


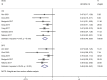
Similar articles
-
Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis.J Gastroenterol Hepatol. 2020 Aug;35(8):1277-1287. doi: 10.1111/jgh.15010. Epub 2020 Mar 3. J Gastroenterol Hepatol. 2020. PMID: 32052876
-
Comparison of Sorafenib versus Hepatic Arterial Infusion Chemotherapy-Based Treatment for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis.Gut Liver. 2021 Mar 15;15(2):284-294. doi: 10.5009/gnl19367. Gut Liver. 2021. PMID: 32307975 Free PMC article.
-
Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial.Radiology. 2022 May;303(2):455-464. doi: 10.1148/radiol.211545. Epub 2022 Feb 1. Radiology. 2022. PMID: 35103539 Clinical Trial.
-
Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis.Cancer Chemother Pharmacol. 2018 Sep;82(3):469-478. doi: 10.1007/s00280-018-3638-0. Epub 2018 Jul 7. Cancer Chemother Pharmacol. 2018. PMID: 29982870 Clinical Trial.
-
Comparative efficacy and safety of multimodality treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: patient-level network meta-analysis.Front Oncol. 2024 Feb 16;14:1344798. doi: 10.3389/fonc.2024.1344798. eCollection 2024. Front Oncol. 2024. PMID: 38434681 Free PMC article.
Cited by
-
Efficacy and safety of hepatic arterial infusion chemotherapy combined with immune checkpoint inhibitors and tyrosine kinase inhibitors in advanced hepatocellular carcinoma: A systematic review and meta‑analysis.Oncol Lett. 2023 Oct 30;26(6):534. doi: 10.3892/ol.2023.14121. eCollection 2023 Dec. Oncol Lett. 2023. PMID: 38020293 Free PMC article.
-
Sorafenib with or without co-interventions for hepatocellular carcinoma.Cochrane Database Syst Rev. 2025 Jun 26;6(6):CD015851. doi: 10.1002/14651858.CD015851. Cochrane Database Syst Rev. 2025. PMID: 40568833 Free PMC article. Review.
-
Anticipation for hepatic arterial infusion chemotherapy in the treatment of hepatocellular carcinoma.World J Gastrointest Oncol. 2025 Feb 15;17(2):100505. doi: 10.4251/wjgo.v17.i2.100505. World J Gastrointest Oncol. 2025. PMID: 39958551 Free PMC article.
-
High-Risk Hepatocellular Carcinoma: Hepatic Arterial Infusion Chemotherapy versus Transarterial Chemoembolization.J Hepatocell Carcinoma. 2024 Mar 27;11:651-663. doi: 10.2147/JHC.S455953. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 38559554 Free PMC article.
-
Higher objective responses by hepatic arterial infusion chemotherapy following atezolizumab and bevacizumab failure than when used as initial therapy in hepatocellular carcinoma: a retrospective study.Abdom Radiol (NY). 2024 Sep;49(9):3127-3135. doi: 10.1007/s00261-024-04308-6. Epub 2024 Apr 28. Abdom Radiol (NY). 2024. PMID: 38678485
References
-
- Ascione A, Fontanella L, Imparato M, Rinaldi L, De Luca M. Mortality from cirrhosis and hepatocellular carcinoma in Western Europe over the last 40 years. Liver Int (2017) 37(8):1193–201. - PubMed
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol (2017) 3(4):524–48. doi: 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

