Organization of cervical cancer screening with DNA-HPV testing impact on early-stage cancer detection: a population-based demonstration study in a Brazilian city
- PMID: 36776450
- PMCID: PMC9903591
- DOI: 10.1016/j.lana.2021.100084
Organization of cervical cancer screening with DNA-HPV testing impact on early-stage cancer detection: a population-based demonstration study in a Brazilian city
Abstract
Background: Cervical cancer is a preventable disease, and the Brazilian screening is opportunistic and has low impact. The current study evaluated an initiative to organize screening using DNA-HPV testing as a replacement for cytology.
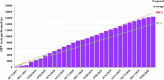
Methods: This demonstration study examined information from 16 384 DNA-HPV tests for screening in women aged 25-64 years from Indaiatuba city between October 2017-March 2020. The comparison was 20 284 women screened using cytology between October 2014-March 2017. The flowchart indicates the repetition of a negative test in five years. HPV16- and/or HPV18-positive tests and the 12 pooled high-risk HPV-positive tests with abnormal liquid-based cytology were referred for colposcopy. If cytology was negative, the HPV test was repeated in 12 months. The analyses evaluated coverage, age-group compliance, and cancer detected.
Findings: After 30 months, the coverage projection was greater than 80%. The age compliance for the HPV test was 99.25%, compared to 78.0% in the cytology program. The HPV test program showed 86.8% negative tests and 6.3% colposcopy referrals, with 78% colposcopies performed. The HPV testing program detected 21 women with cervical cancer with a mean age of 39.6 years, and 67% of cancers were early-stage compared to 12 cervical cancer cases detected by cytological screening (p=0.0284) with a mean age of 49.3 years (p=0.0158), and one case of early-stage (p=0.0014).
Interpretation: Organizing cervical cancer screening using DNA-HPV testing demonstrated high coverage and age compliance in a real-life scenario, and it had an immediate impact on cervical cancer detection at an early-stage.
Funding: University of Campinas, Indaiatuba City, and Roche Diagnostics.
Keywords: Pap smear, DNA-HPV test; Papillomavirus infections; cancer screening; cervical cancer; public health.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Estimate/2020 – Cancer Incidence in Brazil. Instituto Nacional de Cancer Jose Alencar Gomes da Silva (INCA) 2020 https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//... Accessed 22 June 2021.
-
- Vol. 10. IARC Press; Lyon: 2005. IARC. (International Agency for Research on Cancer–WHO: IARC handbook on cervical cancer prevention). Cervix Cancer Screening.
-
- Brazilian Cervical Cancer Screening Guidelines. Instituto Nacional de Cancer Jose Alencar Gomes da Silva (INCA) 2016 http://www.citologiaclinica.org.br/site/pdf/documentos/diretrizes–para–o... Accessed 22 June 2021.
-
- Freitas RA, Carvasan GA, Morais SS, Zeferino LC. Excessive Pap smears due to opportunistic cervical cancer screening. Eur J Gynaecol Oncol. 2008;29:479–482. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

