Effectiveness of Favipiravir monotherapy in the treatment of COVID-19: real world data analysis from Thailand
- PMID: 36776761
- PMCID: PMC9902286
- DOI: 10.1016/j.lansea.2023.100166
Effectiveness of Favipiravir monotherapy in the treatment of COVID-19: real world data analysis from Thailand
Abstract
Background: Previous studies showed that Favipiravir, a selective viral ribonucleic acid dependent-ribonucleic acid polymerase inhibitor, exhibited a trend of clinical improvement within 14 days and promoted viral clearance by day 7, without reduction of mortality rate in COVID-19.
Methods: During the COVID-19 pandemic, Department of Medical Services (Thailand) formulated National Clinical Treatment Guidelines for COVID-19 and approved Favipiravir to eight medical centres. After treatment with Favipiravir monotherapy, we compared real-world data analysis to supportive treatment without antiviral agents.
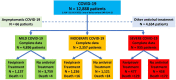
Findings: We analysed 12,888 COVID-19 patients between June 1, 2021, and July 31, 2021. This group study excluded 66 asymptomatic and 4634 COVID-19 patients treated with other antiviral agents. The 4896 mild, 2357 moderate, and 935 severe COVID-19 patients were analysed. All patients neither had previous SARS-CoV-2 infection nor received an mRNA vaccine during study period. Favipiravir monotherapy reduced the 28-day mortality risk in severe COVID-19 by relative risk (RR) = 0.72 (95% CI 0.58-0.91 P = 0.006) after adjustment for aging and hypertension. However, in mild and moderate COVID-19, Favipiravir monotherapy did not significantly reduce 28-day mortality risk by RR = 0.59 (95% CI 0.06-5.43 P = 0.65) after adjustment for aging, and RR = 0.60 (95% CI 0.32-1.13 P = 0.11) after adjustment for aging and obesity, respectively. In the patient with recovery, Favipiravir monotherapy exhibited a shortening time to recovery when compared to supportive treatment without antiviral agents (mean ± SD by 9.6 ± 7.1 vs. 12.9 ± 7.6 days: P < 0.0001, 10.0 ± 5.9 vs. 12.4 ± 5.3 days: P < 0.0001, and 11.2 ± 7.8 vs. 13.1 ± 8.0 days: P < 0.0001 in mild, moderate, and severe COVID-19 respectively).
Interpretation: Real-world data analysis showed that favipiravir monotherapy was superior to supportive treatment without antiviral agents in shortening the recovery time in surviving patients and significantly reducing 28-day mortality risk in severe COVID-19.
Funding: Department of Medical Services, Ministry of Public Health, Thailand.
Keywords: COVID-19; Favipiravir; Molnupiravir; Mortality rate; Nirmatrelvir; Paxlovid; Remdesivir; Time to recovery.
© 2023 The Author(s).
Conflict of interest statement
BM has received honoraria and travel reimbursement from Lundbeck, Pfizer, and Servier. NM has received travel reimbursement from Lundbeck and Pfizer. AS, ST, SA, and SK report no conflicts of interest in this work. Narong Maneeton and Benchalak Maneeton are husband and wife. Benchalak Maneeton is the sister of Subsai Kongsaengdao. The funders had no role in study design, data collection or analysis, preparation of the manuscript or decision to publish.
Figures

References
-
- THAI NATIONAL GUIDELINE for COVID-19 . 2021. Guidelines for health care and public health workers.https://ddc.moph.go.th/viralpneumonia/eng/guidelines.php
LinkOut - more resources
Full Text Sources
Miscellaneous

