Field evaluation of the novel One Step Malaria Pf and Pf/Pv rapid diagnostic tests and the proportion of HRP-2 gene deletion identified on samples collected in the Pwani region, Tanzania
- PMID: 36776799
- PMCID: PMC9904258
- DOI: 10.1186/s42269-023-00992-4
Field evaluation of the novel One Step Malaria Pf and Pf/Pv rapid diagnostic tests and the proportion of HRP-2 gene deletion identified on samples collected in the Pwani region, Tanzania
Abstract
Background: Malaria rapid diagnostic tests (mRDTs) have played an important role in the early detection of clinical malaria in an endemic area. While several mRDTs are currently on the market, the availability of mRDTs with high sensitivity and specificity will merit the fight against malaria. We evaluated the field performance of a novel One Step Malaria (P.f/P.v) Tri-line and One Step Malaria (P.f) rapid test kits in Pwani, Tanzania.
Methods: In a cross-sectional study conducted in Bagamoyo and Kibiti districts in Tanzania, symptomatic patients were tested using the SD BIOLINE, One Step Malaria (P.f/P.v) Tri-line and One Step Malaria (P.f) rapid test kits, microscope, and quantitative Polymerase Chain Reaction (qPCR). An additional qPCR assay was carried out to detect Histidine-Rich Protein 2 (HRP-2) gene deletion on mRDT negative but microscope and qPCR positive samples. Microscope results confirmed by qPCR were used for analysis, where qPCR was used as a reference method.
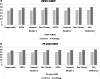
Results: The sensitivity and specificity of One Step P.f/P.v Tri-line mRDTs were 96.0% (CI 93.5-97.7%) and 98.3% (CI 96.8-99.2%), respectively. One Step P.f mRDT had sensitivity and specificity of 95.2% (CI 92.5-97.1%) and 97.9% (CI 96.3-99.0%) respectively. Positive predictive value (PPV) was 97.6% (CI 95.4-98.7%) and negative predictive value (NPV) was 96.2% (CI 95.5-98.3%) for the One Step P.f/P.v Tri-line mRDTs respectively, while One Step P.f mRDT had positive predictive value (PPV) and negative predictive value (NPV) of 97.0% (CI 94.8-98.3%) and 96.7 (CI 94.9-97.9%) respectively. 9.8% (CI 7.84-11.76) of all samples tested and reported to be malaria-negative by mRDT had HRP-2 gene deletion.
Conclusion: One Step Malaria P.f/P.v Tri-line and One Step Malaria P.f rapid test kits have similar sensitivity and specificity as the standard mRDT that is currently in the market, demonstrating the potential to contribute in the fight against malaria in endemic settings. However, the identified malaria parasites population with HRP-2 gene deletion pose a threat to the current mRDT usability in the field and warrants further investigations.
Keywords: HRP-2 gene deletion; Malaria; Rapid diagnostic test; Sensitivity; Specificity.
© The Author(s) 2023.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
Similar articles
-
Field evaluation of a malaria rapid diagnostic test (ICT Pf).S Afr Med J. 2009 Nov;99(11):810-3. S Afr Med J. 2009. PMID: 20218482
-
Performance evaluation of the highly sensitive histidine-rich protein 2 rapid test for plasmodium falciparum malaria in North-West Tanzania.Malar J. 2021 Jan 22;20(1):58. doi: 10.1186/s12936-020-03568-z. Malar J. 2021. PMID: 33482835 Free PMC article.
-
Malaria rapid diagnostic tests for the case management of febrile children in Nigerian primary healthcare settings: a cross-sectional study.J Egypt Public Health Assoc. 2022 May 12;97(1):10. doi: 10.1186/s42506-022-00105-5. J Egypt Public Health Assoc. 2022. PMID: 35551536 Free PMC article.
-
Malaria rapid diagnostic test evaluation at private retail pharmacies in Kumasi, Ghana.J Res Pharm Pract. 2016 Jul-Sep;5(3):175-80. doi: 10.4103/2279-042X.185723. J Res Pharm Pract. 2016. PMID: 27512708 Free PMC article.
-
Diagnostic Accuracy of CareStart™ Malaria HRP2 and SD Bioline Pf/PAN for Malaria in Febrile Outpatients in Varying Malaria Transmission Settings in Cameroon.Diagnostics (Basel). 2021 Aug 27;11(9):1556. doi: 10.3390/diagnostics11091556. Diagnostics (Basel). 2021. PMID: 34573898 Free PMC article.
Cited by
-
Plasmodium falciparum gametocyte burden in a Tanzanian heterogeneous transmission setting.Malar J. 2025 Feb 21;24(1):54. doi: 10.1186/s12936-025-05270-4. Malar J. 2025. PMID: 39985008 Free PMC article.
-
Entomological survey of sibling species in the Anopheles funestus group in Tanzania confirms the role of Anopheles parensis as a secondary malaria vector.Parasit Vectors. 2024 Jun 17;17(1):261. doi: 10.1186/s13071-024-06348-9. Parasit Vectors. 2024. PMID: 38886827 Free PMC article.
-
Plasmodium falciparum with pfhrp2 and pfhrp3 gene deletions in asymptomatic malaria infections in the Lake Victoria region, Kenya.Trop Med Health. 2024 Dec 18;52(1):94. doi: 10.1186/s41182-024-00664-7. Trop Med Health. 2024. PMID: 39696727 Free PMC article.
-
Deletions of Histidine-Rich Protein 2/3 Genes in Natural Plasmodium falciparum Populations from Cameroon and India: Role of Asymptomatic and Submicroscopic Infections.Am J Trop Med Hyg. 2024 Apr 30;110(6):1100-1109. doi: 10.4269/ajtmh.23-0896. Print 2024 Jun 5. Am J Trop Med Hyg. 2024. PMID: 38688260 Free PMC article.
References
-
- Agaba BB, Yeka A, Nsobya S, Arinaitwe E, Nankabirwa J, Opigo J, Mbaka P, Lim CS, Kalyango JN, Karamagi C, Kamya MR. Systematic review of the status of pfhrp2 and pfhrp3 gene deletion, approaches and methods used for its estimation and reporting in Plasmodium falciparum populations in Africa: review of published studies 2010–2019. Malar J. 2019;18(1):1–10. doi: 10.1186/s12936-019-2987-4. - DOI - PMC - PubMed
-
- Charpentier E, Benichou E, Pagès A, Chauvin P, Fillaux J, Valentin A, Guegan H, Guemas E, Salabert AS, Armengol C, Menard S, Cassaing S, Berry A, Iriart X. Performance evaluation of different strategies based on microscopy techniques, rapid diagnostic test and molecular loop-mediated isothermal amplification assay for the diagnosis of imported malaria. Clin Microbiol Infect. 2020;26(1):115–121. doi: 10.1016/j.cmi.2019.05.010. - DOI - PubMed
LinkOut - more resources
Full Text Sources
