Immunoinformatics design of multivalent epitope vaccine against monkeypox virus and its variants using membrane-bound, enveloped, and extracellular proteins as targets
- PMID: 36776835
- PMCID: PMC9908764
- DOI: 10.3389/fimmu.2023.1091941
Immunoinformatics design of multivalent epitope vaccine against monkeypox virus and its variants using membrane-bound, enveloped, and extracellular proteins as targets
Abstract
Introduction: The current monkeypox (MPX) outbreak, caused by the monkeypox virus (MPXV), has turned into a global concern, with over 59,000 infection cases and 23 deaths worldwide.
Objectives: Herein, we aimed to exploit robust immunoinformatics approach, targeting membrane-bound, enveloped, and extracellular proteins of MPXV to formulate a chimeric antigen. Such a strategy could similarly be applied for identifying immunodominant epitopes and designing multi-epitope vaccine ensembles in other pathogens responsible for chronic pathologies that are difficult to intervene against.
Methods: A reverse vaccinology pipeline was used to select 11 potential vaccine candidates, which were screened and mapped to predict immunodominant B-cell and T-cell epitopes. The finalized epitopes were merged with the aid of suitable linkers, an adjuvant (Resuscitation-promoting factor), a PADRE sequence (13 aa), and an HIV TAT sequence (11 aa) to formulate a multivalent epitope vaccine. Bioinformatics tools were employed to carry out codon adaptation and computational cloning. The tertiary structure of the chimeric vaccine construct was modeled via I-TASSER, and its interaction with Toll-like receptor 4 (TLR4) was evaluated using molecular docking and molecular dynamics simulation. C-ImmSim server was implemented to examine the immune response against the designed multi-epitope antigen.
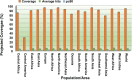
Results and discussion: The designed chimeric vaccine construct included 21 immunodominant epitopes (six B-cell, eight cytotoxic T lymphocyte, and seven helper T-lymphocyte) and is predicted non-allergen, antigenic, soluble, with suitable physicochemical features, that can promote cross-protection among the MPXV strains. The selected epitopes indicated a wide global population coverage (93.62%). Most finalized epitopes have 70%-100% sequence similarity with the experimentally validated immune epitopes of the vaccinia virus, which can be helpful in the speedy progression of vaccine design. Lastly, molecular docking and molecular dynamics simulation computed stable and energetically favourable interaction between the putative antigen and TLR4.
Conclusion: Our results show that the multi-epitope vaccine might elicit cellular and humoral immune responses and could be a potential vaccine candidate against the MPXV infection. Further experimental testing of the proposed vaccine is warranted to validate its safety and efficacy profile.
Keywords: T-and B-cell; immunoinformatics; monkeypox; monkeypox virus; multi-epitope vaccine; multivalent epitope vaccine; reverse vaccinology.
Copyright © 2023 Waqas, Aziz, Liò, Khan, Ali, Iqbal, Khan and Almajhdi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Employing an immunoinformatics approach revealed potent multi-epitope based subunit vaccine for lymphocytic choriomeningitis virus.J Infect Public Health. 2023 Feb;16(2):214-232. doi: 10.1016/j.jiph.2022.12.023. Epub 2022 Dec 31. J Infect Public Health. 2023. PMID: 36603375
-
Contriving multi-epitope vaccine ensemble for monkeypox disease using an immunoinformatics approach.Front Immunol. 2022 Oct 13;13:1004804. doi: 10.3389/fimmu.2022.1004804. eCollection 2022. Front Immunol. 2022. PMID: 36311762 Free PMC article.
-
Multi-epitope chimeric vaccine design against emerging Monkeypox virus via reverse vaccinology techniques- a bioinformatics and immunoinformatics approach.Front Immunol. 2022 Aug 25;13:985450. doi: 10.3389/fimmu.2022.985450. eCollection 2022. Front Immunol. 2022. PMID: 36091024 Free PMC article.
-
Immunogenic proteins and potential delivery platforms for mpox virus vaccine development: A rapid review.Int J Biol Macromol. 2023 Aug 1;245:125515. doi: 10.1016/j.ijbiomac.2023.125515. Epub 2023 Jun 21. Int J Biol Macromol. 2023. PMID: 37353117 Free PMC article. Review.
-
Mpox virus (MPXV): comprehensive analysis of pandemic risks, pathophysiology, treatments, and mRNA vaccine development.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6143-6163. doi: 10.1007/s00210-024-03649-9. Epub 2025 Jan 8. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39777535 Review.
Cited by
-
Immunoinformatics and reverse vaccinology approach in designing a novel highly immunogenic multivalent peptide-based vaccine against the human monkeypox virus.Front Mol Biosci. 2023 Nov 22;10:1295817. doi: 10.3389/fmolb.2023.1295817. eCollection 2023. Front Mol Biosci. 2023. PMID: 38074091 Free PMC article.
-
Design of a multi-epitope vaccine against goatpox virus using an immunoinformatics approach.Front Cell Infect Microbiol. 2024 Feb 29;13:1309096. doi: 10.3389/fcimb.2023.1309096. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38487680 Free PMC article.
-
Designing a multi-epitope subunit vaccine against Orf virus using molecular docking and molecular dynamics.Virulence. 2024 Dec;15(1):2398171. doi: 10.1080/21505594.2024.2398171. Epub 2024 Sep 11. Virulence. 2024. PMID: 39258802 Free PMC article.
-
Human monkeypox disease prediction using novel modified restricted Boltzmann machine-based equilibrium optimizer.Sci Rep. 2024 Jul 30;14(1):17612. doi: 10.1038/s41598-024-68836-3. Sci Rep. 2024. PMID: 39080387 Free PMC article.
-
Multi-epitope vaccines: charting a new frontier in monkeypox prevention and control.Hum Cell. 2025 Jul 9;38(5):126. doi: 10.1007/s13577-025-01255-2. Hum Cell. 2025. PMID: 40632349 Review.
References
-
- W. H. Organization . Monkeypox outbreak: Global trends. (2022). Available: https://worldhealthorg.shinyapps.io/mpx_global/#1_Overview [Accessed 15 September 2022]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

