Extensive blood transcriptome analysis reveals cellular signaling networks activated by circulating glycocalyx components reflecting vascular injury in COVID-19
- PMID: 36776845
- PMCID: PMC9909741
- DOI: 10.3389/fimmu.2023.1129766
Extensive blood transcriptome analysis reveals cellular signaling networks activated by circulating glycocalyx components reflecting vascular injury in COVID-19
Abstract
Background: Degradation of the endothelial protective glycocalyx layer during COVID-19 infection leads to shedding of major glycocalyx components. These circulating proteins and their degradation products may feedback on immune and endothelial cells and activate molecular signaling cascades in COVID-19 associated microvascular injury. To test this hypothesis, we measured plasma glycocalyx components in patients with SARS-CoV-2 infection of variable disease severity and identified molecular signaling networks activated by glycocalyx components in immune and endothelial cells.
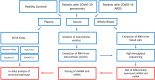
Methods: We studied patients with RT-PCR confirmed COVID-19 pneumonia, patients with COVID-19 Acute Respiratory Distress Syndrome (ARDS) and healthy controls (wildtype, n=20 in each group) and measured syndecan-1, heparan sulfate and hyaluronic acid. The in-silico construction of signaling networks was based on RNA sequencing (RNAseq) of mRNA transcripts derived from blood cells and of miRNAs isolated from extracellular vesicles from the identical cohort. Differentially regulated RNAs between groups were identified by gene expression analysis. Both RNAseq data sets were used for network construction of circulating glycosaminoglycans focusing on immune and endothelial cells.
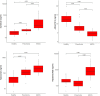
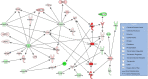
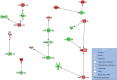
Results: Plasma concentrations of glycocalyx components were highest in COVID-19 ARDS. Hyaluronic acid plasma levels in patients admitted with COVID-19 pneumonia who later developed ARDS during hospital treatment (n=8) were significantly higher at hospital admission than in patients with an early recovery. RNAseq identified hyaluronic acid as an upregulator of TLR4 in pneumonia and ARDS. In COVID-19 ARDS, syndecan-1 increased IL-6, which was significantly higher than in pneumonia. In ARDS, hyaluronic acid activated NRP1, a co-receptor of activated VEGFA, which is associated with pulmonary vascular hyperpermeability and interacted with VCAN (upregulated), a proteoglycan important for chemokine communication.
Conclusions: Circulating glycocalyx components in COVID-19 have distinct biologic feedback effects on immune and endothelial cells and result in upregulation of key regulatory transcripts leading to further immune activation and more severe systemic inflammation. These consequences are most pronounced during the early hospital phase of COVID-19 before pulmonary failure develops. Elevated levels of circulating glycocalyx components may early identify patients at risk for microvascular injury and ARDS. The timely inhibition of glycocalyx degradation could provide a novel therapeutic approach to prevent the development of ARDS in COVID-19.
Keywords: COVID-19; acute respiratory distress syndrome; cell-free microRNAs; endothelial dysfunction; extracellular vesicles; glycocalyx; small RNA.
Copyright © 2023 Borrmann, Brandes, Kirchner, Klein, Billaud, Reithmair, Rehm, Schelling, Pfaffl and Meidert.
Conflict of interest statement
JB is an employee at Qiagen and Qiagen products were used in this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Extracellular Vesicle Associated miRNAs Regulate Signaling Pathways Involved in COVID-19 Pneumonia and the Progression to Severe Acute Respiratory Corona Virus-2 Syndrome.Front Immunol. 2021 Dec 9;12:784028. doi: 10.3389/fimmu.2021.784028. eCollection 2021. Front Immunol. 2021. PMID: 34956213 Free PMC article.
-
Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients.Life Sci. 2021 Jul 1;276:119376. doi: 10.1016/j.lfs.2021.119376. Epub 2021 Mar 27. Life Sci. 2021. PMID: 33781826 Free PMC article.
-
Endothelial glycocalyx degradation in multisystem inflammatory syndrome in children related to COVID-19.J Mol Med (Berl). 2022 May;100(5):735-746. doi: 10.1007/s00109-022-02190-7. Epub 2022 Mar 26. J Mol Med (Berl). 2022. PMID: 35347344 Free PMC article.
-
Vascular endothelial injury exacerbates coronavirus disease 2019: The role of endothelial glycocalyx protection.Microcirculation. 2021 Apr;28(3):e12654. doi: 10.1111/micc.12654. Epub 2020 Aug 30. Microcirculation. 2021. PMID: 32791568 Free PMC article. Review.
-
Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19.Biomed J. 2020 Oct;43(5):399-413. doi: 10.1016/j.bj.2020.08.007. Epub 2020 Aug 24. Biomed J. 2020. PMID: 33032965 Free PMC article. Review.
Cited by
-
Low-Frequency Ventilation May Facilitate Weaning in Acute Respiratory Distress Syndrome Treated with Extracorporeal Membrane Oxygenation: A Randomized Controlled Trial.J Clin Med. 2024 Aug 27;13(17):5094. doi: 10.3390/jcm13175094. J Clin Med. 2024. PMID: 39274307 Free PMC article.
-
Therapeutic strategies targeting the endothelial glycocalyx.Curr Opin Clin Nutr Metab Care. 2023 Nov 1;26(6):543-550. doi: 10.1097/MCO.0000000000000973. Epub 2023 Aug 9. Curr Opin Clin Nutr Metab Care. 2023. PMID: 37555800 Free PMC article. Review.
-
MicroRNAs Derived from Extracellular Vesicles: Keys to Understanding SARS-CoV-2 Vaccination Response in Cancer Patients?Cancers (Basel). 2023 Aug 8;15(16):4017. doi: 10.3390/cancers15164017. Cancers (Basel). 2023. PMID: 37627045 Free PMC article. Review.
-
Extracellular DNA, hyaluronic acid, HIF pathways, and LncRNAs as predictive biomarkers of severe COVID-19.Virol J. 2025 Jul 23;22(1):252. doi: 10.1186/s12985-025-02886-5. Virol J. 2025. PMID: 40702494 Free PMC article. Review.
-
Micro- and Macrovascular Effects of Inflammation in Peripheral Artery Disease-Pathophysiology and Translational Therapeutic Approaches.Biomedicines. 2023 Aug 17;11(8):2284. doi: 10.3390/biomedicines11082284. Biomedicines. 2023. PMID: 37626780 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

