Characterization of myeloperoxidase and its contribution to antimicrobial effect on extracellular traps in flounder (Paralichthys olivaceus)
- PMID: 36776890
- PMCID: PMC9908613
- DOI: 10.3389/fimmu.2023.1124813
Characterization of myeloperoxidase and its contribution to antimicrobial effect on extracellular traps in flounder (Paralichthys olivaceus)
Abstract
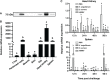
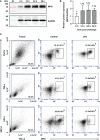
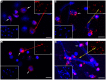
Myeloperoxidase (MPO) is a cationic leukocyte haloperoxidase and together with other proteins, they possess activities against various microorganisms and are involved in extracellular trap (ET) formation. The present work describes the gene and deduced protein sequences, and functions of MPO in flounder (PoMPO). The PoMPO possesses a 2313 bp open reading frame (ORF) that encodes a protein of 770 amino acids. The highest PoMPO mRNA expression levels were found in the head kidney, followed by peritoneal cells, gill, spleen, skin, muscle, and liver. PoMPO was expressed in MHCII+ and GCSFR+ cells which indicated that PoMPO mainly is expressed in flounder macrophages and granulocytes. Bacterial lipopolysaccharide-stimulated peritoneal leukocytes showed an increased protein level of PoMPO while it seemed that LPS also promoted the migration of MPO+ cells from the head kidney into the peripheral blood and peritoneal cavity. After phorbol 12-myristate 13-acetate (PMA) or bacterial stimulation, flounder leukocytes produced typical ET structures containing DNA with decoration by MPO. The ETs containing DNA and PoMPO effectively inhibited the proliferation of ET-trapped bacteria. Blocking PoMPO with antibodies decreased the enzymatic activity, which attenuated the antibacterial activity of ETs. This study pinpoints the involvement of ETs in flounder innate responses to pathogens.
Keywords: antibiosis; extracellular traps; fish; immune response; myeloperoxidase.
Copyright © 2023 Gan, Chi, Dalmo, Meng, Tang, Xing, Sheng and Zhan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Ontogeny of myeloperoxidase (MPO) positive cells in flounder (Paralichthys olivaceus).Mol Immunol. 2024 Jun;170:26-34. doi: 10.1016/j.molimm.2024.04.005. Epub 2024 Apr 10. Mol Immunol. 2024. PMID: 38603988
-
G-CSF modulates innate and adaptive immunity via the ligand-receptor pathway of binding GCSFR in flounder (Paralichthys olivaceus).Fish Shellfish Immunol. 2025 Mar;158:110160. doi: 10.1016/j.fsi.2025.110160. Epub 2025 Jan 27. Fish Shellfish Immunol. 2025. PMID: 39880324
-
Interleukin-2 (IL-2) in flounder (Paralichthys olivaceus): Molecular cloning, characterization and bioactivity analysis.Fish Shellfish Immunol. 2019 Oct;93:55-65. doi: 10.1016/j.fsi.2019.07.023. Epub 2019 Jul 15. Fish Shellfish Immunol. 2019. PMID: 31319204
-
Molecular cloning and characterization of interferon regulatory factor 9 (IRF9) in Japanese flounder, Paralichthys olivaceus.Fish Shellfish Immunol. 2014 Aug;39(2):138-44. doi: 10.1016/j.fsi.2014.05.002. Epub 2014 May 14. Fish Shellfish Immunol. 2014. PMID: 24837327
-
Matrix metalloproteinase 9 modulates immune response along with the formation of extracellular traps in flounder (Paralichthys olivaceus).Fish Shellfish Immunol. 2023 Feb;133:108570. doi: 10.1016/j.fsi.2023.108570. Epub 2023 Jan 28. Fish Shellfish Immunol. 2023. PMID: 36717064
Cited by
-
Dietary Chlorella vulgaris supplementation modulates health, microbiota and the response to oxidative stress of Atlantic salmon.Sci Rep. 2024 Oct 10;14(1):23674. doi: 10.1038/s41598-024-72531-8. Sci Rep. 2024. PMID: 39389986 Free PMC article.
-
Unraveling the Bioactivities and Immunomodulatory Potential of Postbiotics Derived from Bacillus subtilis and B. amyloliquefaciens for Aquaculture.Probiotics Antimicrob Proteins. 2025 Apr 4. doi: 10.1007/s12602-025-10528-z. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40186049
-
Transcriptome Analysis of Peritoneal Cells Reveals the Early Immune Response of Flounder (Paralichthys olivaceus) to Inactivated Vibrio anguillarum Immunization.Vaccines (Basel). 2023 Oct 16;11(10):1603. doi: 10.3390/vaccines11101603. Vaccines (Basel). 2023. PMID: 37897005 Free PMC article.
-
Pentatrichomonas hominis induces extracellular traps formation of macrophages via the TLR2/NADPH/PAD4 pathway.Parasit Vectors. 2025 Jul 1;18(1):248. doi: 10.1186/s13071-025-06840-w. Parasit Vectors. 2025. PMID: 40598534 Free PMC article.
-
A Bacterial Myeloperoxidase with Antimicrobial Properties.BioTech (Basel). 2023 May 5;12(2):33. doi: 10.3390/biotech12020033. BioTech (Basel). 2023. PMID: 37218750 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

