Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream
- PMID: 36776937
- PMCID: PMC9910099
- DOI: 10.3390/neuroglia3010003
Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream
Abstract
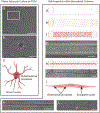
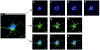
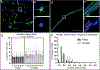
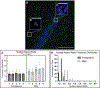
Neural precursor cells (NPCs) are generated in the subventricular zone (SVZ) and travel through the rostral migratory stream (RMS) to replace olfactory bulb interneurons in the brains of most adult mammals. Following brain injury, SVZ-derived NPCs can divert from the RMS and migrate toward injured brain regions but arrive in numbers too low to promote functional recovery without experimental intervention. Our lab has biofabricated a "living scaffold" that replicates the structural and functional features of the endogenous RMS. This tissue-engineered rostral migratory stream (TE-RMS) is a new regenerative medicine strategy designed to facilitate stable and sustained NPC delivery into neuron-deficient brain regions following brain injury or neurodegenerative disease and an in vitro tool to investigate the mechanisms of neuronal migration and cell-cell communication. We have previously shown that the TE-RMS replicates the basic structure and protein expression of the endogenous RMS and can direct immature neuronal migration in vitro and in vivo. Here, we further describe profound morphological changes that occur following precise physical manipulation and subsequent self-assembly of astrocytes into the TE-RMS, including significant cytoskeletal rearrangement and nuclear elongation. The unique cytoskeletal and nuclear architecture of TE-RMS astrocytes mimics astrocytes in the endogenous rat RMS. Advanced imaging techniques reveal the unique morphology of TE-RMS cells that has yet to be described of astrocytes in vitro. The TE-RMS offers a novel platform to elucidate astrocyte cytoskeletal and nuclear dynamics and their relationship to cell behavior and function.
Keywords: astrocyte; cell morphology; cytoskeleton; nuclear morphology; nucleus; rostral migratory stream; tissue engineering.
Conflict of interest statement
Conflicts of Interest: There are no other potential conflicts of interest to disclose.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
