Impact of exogenous hydrogen peroxide on osteogenic differentiation of broiler chicken compact bones derived mesenchymal stem cells
- PMID: 36776980
- PMCID: PMC9909420
- DOI: 10.3389/fphys.2023.1124355
Impact of exogenous hydrogen peroxide on osteogenic differentiation of broiler chicken compact bones derived mesenchymal stem cells
Abstract
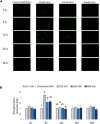
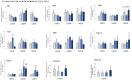
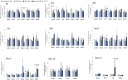
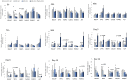
The effects of hydrogen peroxide (H2O2) on the osteogenic differentiation of primary chicken mesenchymal stem cells (MSCs) were investigated. MSCs were subjected to an osteogenic program and exposed to various concentrations of H2O2 for 14 days. Results showed that high concentrations of H2O2 (200 and 400 nM) significantly increased pro-apoptotic marker CASP8 expression and impaired osteogenic differentiation, as indicated by decreased mRNA expression levels of osteogenesis-related genes and reduced in vitro mineralization. In contrast, long-term H2O2 exposure promoted basal expression of adipogenic markers at the expense of osteogenesis in MSCs during osteogenic differentiation, and increased intracellular reactive oxygen species (ROS) production, as well as altered antioxidant enzyme gene expression. These findings suggest that long-term H2O2-induced ROS production impairs osteogenic differentiation in chicken MSCs under an osteogenic program.
Keywords: bone health; cell differentiation; cellular ROS; chicken MSCs; oxidative stress.
Copyright © 2023 Tompkins, Liu and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Effect of 20(S)-Hydroxycholesterol on Multilineage Differentiation of Mesenchymal Stem Cells Isolated from Compact Bones in Chicken.Genes (Basel). 2020 Nov 17;11(11):1360. doi: 10.3390/genes11111360. Genes (Basel). 2020. PMID: 33213081 Free PMC article.
-
Effects of l-Tryptophan and 1,25-Dihydroxycholecalciferol on Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells Isolated from the Compact Bones of Broilers and Layers.J Agric Food Chem. 2022 Aug 31;70(34):10476-10489. doi: 10.1021/acs.jafc.2c03451. Epub 2022 Aug 22. J Agric Food Chem. 2022. PMID: 35993842
-
Vitamin D3 contributes to enhanced osteogenic differentiation of MSCs under oxidative stress condition via activating the endogenous antioxidant system.Osteoporos Int. 2018 Aug;29(8):1917-1926. doi: 10.1007/s00198-018-4547-0. Epub 2018 Jun 2. Osteoporos Int. 2018. PMID: 29860665
-
Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation.Int J Mol Sci. 2021 Dec 15;22(24):13458. doi: 10.3390/ijms222413458. Int J Mol Sci. 2021. PMID: 34948255 Free PMC article.
-
Ebselen rescues oxidative-stress-suppressed osteogenic differentiation of bone-marrow-derived mesenchymal stem cells via an antioxidant effect and the PI3K/Akt pathway.J Trace Elem Med Biol. 2019 Sep;55:64-70. doi: 10.1016/j.jtemb.2019.06.002. Epub 2019 Jun 10. J Trace Elem Med Biol. 2019. PMID: 31345368
Cited by
-
Effects of Methionine Supplementation Levels in Normal or Reduced Protein Diets on the Body Composition and Femur Bone Characteristics of Broilers Challenged with Coccidia.Animals (Basel). 2024 Mar 16;14(6):917. doi: 10.3390/ani14060917. Animals (Basel). 2024. PMID: 38540016 Free PMC article.
-
The Functional Roles of Methionine and Arginine in Intestinal and Bone Health of Poultry: Review.Animals (Basel). 2023 Sep 18;13(18):2949. doi: 10.3390/ani13182949. Animals (Basel). 2023. PMID: 37760349 Free PMC article. Review.
References
-
- Ali Hassan N., Li Z. (2021). Oxidative stress in broiler chicken and its consequences on meat quality. Int. J. Life Sci. Res. Archive 1 (1), 045–054. 10.53771/ijlsra.2021.1.1.0054 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

