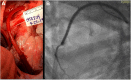
1-Year Patency of Biorestorative Polymeric Coronary Artery Bypass Grafts in an Ovine Model
- PMID: 36777172
- PMCID: PMC9911320
- DOI: 10.1016/j.jacbts.2022.06.021
1-Year Patency of Biorestorative Polymeric Coronary Artery Bypass Grafts in an Ovine Model
Abstract
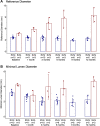
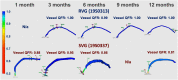
Many attempts have been made to inhibit or counteract saphenous vein graft (SVG) failure modes; however, only external support for SVGs has gained momentum in clinical utility. This study revealed the feasibility of implantation, and showed good patency out to 12 months of the novel biorestorative graft, in a challenging ovine coronary artery bypass graft model. This finding could trigger the first-in-man trial of using the novel material instead of SVG. We believe that, eventually, this novel biorestorative bypass graft can be one of the options for coronary artery bypass graft patients who have difficulty harvesting SVG.
Keywords: CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; IH, intimal hyperplasia; LAD, left anterior descending artery; OCT, optical coherence tomography; QCA, quantitative coronary angiography; QFR, quantitative flow ratio; RVG, restorative vascular graft; SVG, saphenous vein graft; coronary artery bypass graft; coronary artery disease; coronary revascularization; ePTFE, expanded polytetrafluoroethylene; polymeric bypass graft; preclinical model; quantitative flow ratio; restorative vascular graft.
© 2023 The Authors.
Conflict of interest statement
The work described in this paper was fully funded by Xeltis BV. Drs El-Kurdi, Reinöhl, and Cox are all employees of Xeltis. Dr Virmani has received personal fees from Xeltis, and Medtronic; as well as Institutional grants from Abbott Vascular, Boston Scientific, Medtronic, Xeltis and Becton Dickinson. Prof. Onuma has received institutional research grants related to his work as the Chairman of cardiovascular imaging core labs of several clinical trials and registries sponsored by industry, for which he receives no direct compensation. Prof. Serruys has received personal fees from Sino Medical Sciences Technology, Philips/Volcano and Xeltis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures







References
-
- Taggart D.P., Ben Gal Y., Lees B., et al. A randomized trial of external stenting for saphenous vein grafts in coronary artery bypass grafting. Ann Thorac Surg. 2015;99:2039–2045. - PubMed
-
- Mack M.J., Squiers J.J., Lytle B.W., DiMaio J.M., Mohr F.W. Myocardial revascularization surgery: JACC historical breakthroughs in perspective. J Am Coll Cardiol. 2021;78:365–383. - PubMed
-
- Lytle B.W., Blackstone E.H., Sabik J.F., Houghtaling P., Loop F.D., Cosgrove D.M. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. 2004;78:2005–2012. [discussion 2012-2014] - PubMed
-
- Ojha M., Cobbold R.S., Johnston K.W. Influence of angle on wall shear stress distribution for an end-to-side anastomosis. J Vasc Surg. 1994;19:1067–1073. - PubMed
LinkOut - more resources
Full Text Sources

