Tie2-Cre-Induced Inactivation of Non-Nuclear Estrogen Receptor-α Signaling Abrogates Estrogen Protection Against Vascular Injury
- PMID: 36777173
- PMCID: PMC9911321
- DOI: 10.1016/j.jacbts.2022.07.001
Tie2-Cre-Induced Inactivation of Non-Nuclear Estrogen Receptor-α Signaling Abrogates Estrogen Protection Against Vascular Injury
Abstract
Using the Cre-loxP system, we generated the first mouse model in which estrogen receptor-α non-nuclear signaling was inactivated in endothelial cells. Estrogen protection against mechanical vascular injury was impaired in this model. This result indicates the pivotal role of endothelial estrogen receptor-α non-nuclear signaling in the vasculoprotective effects of estrogen.
Keywords: E2, 17β-estradiol; ECGM, endothelial cell growth medium; ER, estrogen receptor; ERαKI/KI, estrogen receptor-αknock-in/knock-in; LVEDD, left ventricular end-diastolic diameter; NOS, nitric oxide synthase; PI3K, phosphatidylinositol 3-kinase; PLA, proximity ligation assay; Vo2, oxygen consumption; cDNA, complementary deoxyribonucleic acid; eNOS, endothelial nitric oxide synthase; endothelial cells; estrogen receptor-α; non-nuclear signaling; tissue-specific regulation.
© 2023 The Authors.
Conflict of interest statement
This work was supported by the Japan Foundation for Applied Enzymology, Japan Heart Foundation Research Grant, SENSHIN Medical Research Foundation, Kobayashi Foundation (Dr Takimoto), Takeda Science Foundation (Drs Ueda and Takimoto), National Institutes of Health HL 052233, National Institutes of Health HL 136962, DK 062729 (Dr Liao), AHA Northeast Research Consortium Postdoctoral Fellowship, Uehara Research Fellowship (Dr Hiroi), and Tri-Service General Hospital Medical Research Foundation Grant TSGH-PH-E-111017 (Dr Liu). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

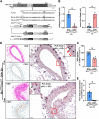
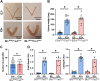


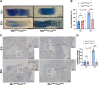

References
-
- Grodstein F., Manson J.E., Colditz G.A., Willett W.C., Speizer F.E., Stampfer M.J. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. - PubMed
-
- Atsma F., Bartelink M.L., Grobbee D.E., van der Schouw Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. - PubMed
-
- Stampfer M.J., Colditz G.A., Willett W.C., et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses' health study. N Engl J Med. 1991;325:756–762. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

