Gallium-modified gelatin nanoparticles loaded with quercetin promote skin wound healing via the regulation of bacterial proliferation and macrophage polarization
- PMID: 36777248
- PMCID: PMC9908762
- DOI: 10.3389/fbioe.2023.1124944
Gallium-modified gelatin nanoparticles loaded with quercetin promote skin wound healing via the regulation of bacterial proliferation and macrophage polarization
Abstract
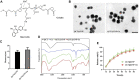
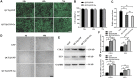
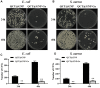
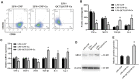
Background: Wound healing is a complicated process involving multiple cell components and can help the re-establishment of the skin's barrier function. Previous studies have pointed out that bacterial infection and sustained inflammatory reactions are the main causes of the delay of wound closure and scar formation during wound healing. The effect of current approaches for scar-free wound repair still faces many challenges, and alternative therapeutic methods are urgently needed to be established. Methods: The basic characteristics of the new-designed nanoparticles were clarified through the characterization of the material. The biocompatibility of the nanoparticles, as well as its effect on fibroblast function, anti-bacterial capacity, inflammation suppressive role, and the underlying mechanism were further verified by a panel of biochemical assays in vitro. Ultimately, pre-clinical rat model was employed to testify its role in wound healing and scar formation in vivo. Results: Firstly, gallium-modified gelatin nanoparticles loaded with quercetin was successfully established, displaying good biocompatibility and facilitative effect on fibroblast function. In addition, the nanoparticles showed prominent anti-bacterial and inflammation-suppressive effects. What's more important, the nanoparticles could also induce the polarization of macrophages from M1 to M2 phenotype to exert its inflammatory inhibitory role through TGF-β/Smad signaling pathway. Ultimately, in vivo experiment showed that the nanoparticles could effectively promote wound repair and inhibit scar formation during the process of wound healing. Conclusion: Taken together, the new nanoparticles have good anti-bacterial and anti-scar formation effects and great potential in the field of skin wound repair, which provides a promising therapeutic strategy for wound treatment.
Keywords: Gallium; Quercetin; macrophage; nanoparticles; wound healing.
Copyright © 2023 Yang, Shi, Yao, Liu and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor, KL, declared a shared affiliation with the authors NS and HL at the time of review.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

