Adalimumab, Infliximab, and Vedolizumab in Treatment of Ulcerative Colitis: A Long-Term Retrospective Study in a Tertiary Referral Center
- PMID: 36777273
- PMCID: PMC9802068
- DOI: 10.1093/crocol/otab049
Adalimumab, Infliximab, and Vedolizumab in Treatment of Ulcerative Colitis: A Long-Term Retrospective Study in a Tertiary Referral Center
Abstract
Background: Biological therapies have changed the landscape of pharmacological management of ulcerative colitis (UC). However, a large proportion of patients do not respond to biologics, lose their response over time, or present adverse drug events. This study aims to assess therapeutic response and treatment persistence to adalimumab, infliximab, and vedolizumab, 3 agents widely used in a tertiary referral center of Saguenay-Lac-Saint-Jean (Quebec, Canada).
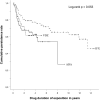
Methods: We conducted a retrospective population-based study with a thorough review of patients' medical charts. Adults at UC diagnosis, with current or past use of adalimumab, infliximab, or vedolizumab, were included in the study. Clinical data were collected in order to assess response phenotypes and persistence to treatment. Kaplan-Meier curves were performed to assess treatment persistence, and predictors for discontinuation were assessed using Cox regression analyses.
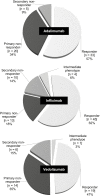
Results: A total of 134 patients were included in this study. For the cases exposed to adalimumab, infliximab, and vedolizumab, 56.9%, 62.5%, and 47.5% were responders, respectively. Mean persistence rates (95% CI) were 5.5 (4.3-6.6), 10.1 (8.7-11.5), and 3.6 (2.9-4.2) years for adalimumab, infliximab, and vedolizumab, respectively. Increased persistence rates were observed in biologic-naïve patients treated with infliximab in comparison to those with the previous exposition to 2 biologics, but no such effect was observed for adalimumab or vedolizumab. Overall, 61.9% of cases had adverse drug events and of these, 6 led to treatment discontinuation.
Conclusion: This study presents long-term treatment persistence data with adalimumab, infliximab, and vedolizumab, showing that more than half of cases treated with these biologics remained on treatment at least 24 months after initiation.
Keywords: adalimumab; infliximab; population-based study; ulcerative colitis; vedolizumab.
© The Author(s) 2021. Published by Oxford University Press on behalf of Crohn\'s & Colitis Foundation.
Figures
References
-
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. . ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413. - PubMed
-
- Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology . Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–23; quiz 524. - PubMed
-
- Mao EJ, Hazlewood GS, Kaplan GG, et al. . Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45(1):3–13. - PubMed
-
- Papamichael K, Gils A, Rutgeerts P, et al. . Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21(1):182–197. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
