Covid-19 vaccine effectiveness against general SARS-CoV-2 infection from the omicron variant: A retrospective cohort study
- PMID: 36777314
- PMCID: PMC9910751
- DOI: 10.1371/journal.pgph.0001111
Covid-19 vaccine effectiveness against general SARS-CoV-2 infection from the omicron variant: A retrospective cohort study
Abstract
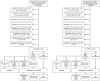
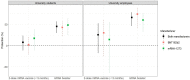
We aim to estimate the effectiveness of 2-dose and 3-dose mRNA vaccination (BNT162b2 and mRNA-1273) against general Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection (asymptomatic or symptomatic) caused by the omicron BA.1 variant. This propensity-score matched retrospective cohort study takes place in a large public university undergoing weekly Coronavirus Disease 2019 (Covid-19) testing in South Carolina, USA. The population consists of 24,145 university students and employees undergoing weekly Covid-19 testing between January 3rd and January 31st, 2022. The analytic sample was constructed via propensity score matching on vaccination status: unvaccinated, completion of 2-dose mRNA series (BNT162b2 or mRNA-1273) within the previous 5 months, and receipt of mRNA booster dose (BNT162b2 or mRNA-1273) within the previous 5 months. The resulting analytic sample consists of 1,944 university students (mean [SD] age, 19.64 [1.42] years, 66.4% female, 81.3% non-Hispanic White) and 658 university employees (mean [SD] age, 43.05 [12.22] years, 64.7% female, 83.3% non-Hispanic White). Booster protection against any SARS-CoV-2 infection was 66.4% among employees (95% CI: 46.1-79.0%; P<.001) and 45.4% among students (95% CI: 30.0-57.4%; P<.001). Compared to the 2-dose mRNA series, estimated increase in protection from the booster dose was 40.8% among employees (P=.024) and 37.7% among students (P=.001). We did not have enough evidence to conclude a statistically significant protective effect of the 2-dose mRNA vaccination series, nor did we have enough evidence to conclude that protection waned in the 5-month period after receipt of the 2nd or 3rd mRNA dose. Furthermore, we did not find evidence that protection varied by manufacturer. We conclude that in adults 18-65 years of age, Covid-19 mRNA booster doses offer moderate protection against general SARS-CoV-2 infection caused by the omicron variant and provide a substantial increase in protection relative to the 2-dose mRNA vaccination series.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study.Lancet Infect Dis. 2023 Apr;23(4):421-434. doi: 10.1016/S1473-3099(22)00732-0. Epub 2022 Dec 12. Lancet Infect Dis. 2023. PMID: 36521506 Free PMC article.
-
Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar.N Engl J Med. 2022 May 12;386(19):1804-1816. doi: 10.1056/NEJMoa2200797. Epub 2022 Mar 9. N Engl J Med. 2022. PMID: 35263534 Free PMC article.
-
Estimated BNT162b2 Vaccine Effectiveness Against Infection With Delta and Omicron Variants Among US Children 5 to 11 Years of Age.JAMA Netw Open. 2022 Dec 1;5(12):e2246915. doi: 10.1001/jamanetworkopen.2022.46915. JAMA Netw Open. 2022. PMID: 36515946 Free PMC article.
-
The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis.Front Public Health. 2022 Jul 13;10:940956. doi: 10.3389/fpubh.2022.940956. eCollection 2022. Front Public Health. 2022. PMID: 35910897 Free PMC article.
-
Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review.Front Immunol. 2022 Aug 24;13:940562. doi: 10.3389/fimmu.2022.940562. eCollection 2022. Front Immunol. 2022. PMID: 36091023 Free PMC article.
Cited by
-
Impact of unequal testing on vaccine effectiveness estimates across two study designs: a simulation study.Nat Commun. 2025 May 24;16(1):4849. doi: 10.1038/s41467-025-59768-1. Nat Commun. 2025. PMID: 40413178 Free PMC article.
-
SARS-CoV-2 variant introduction following spring break travel and transmission mitigation strategies.PLoS One. 2024 May 9;19(5):e0301225. doi: 10.1371/journal.pone.0301225. eCollection 2024. PLoS One. 2024. PMID: 38722935 Free PMC article.
-
An epidemiological modeling framework to inform institutional-level response to infectious disease outbreaks: a Covid-19 case study.Sci Rep. 2024 Mar 27;14(1):7221. doi: 10.1038/s41598-024-57488-y. Sci Rep. 2024. PMID: 38538693 Free PMC article.
-
An epidemiological modeling framework to inform institutional-level response to infectious disease outbreaks: A Covid-19 case study.Res Sq [Preprint]. 2023 Jul 11:rs.3.rs-3116880. doi: 10.21203/rs.3.rs-3116880/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Mar 27;14(1):7221. doi: 10.1038/s41598-024-57488-y. PMID: 37503237 Free PMC article. Updated. Preprint.
-
Preclinical immune efficacy against SARS-CoV-2 beta B.1.351 variant by MVA-based vaccine candidates.Front Immunol. 2023 Dec 12;14:1264323. doi: 10.3389/fimmu.2023.1264323. eCollection 2023. Front Immunol. 2023. PMID: 38155964 Free PMC article.
References
-
- WHO. WHO Coronavirus (COVID-19) Dashboard [Internet]. 2022 [cited 2022 Aug 23]. Available from: https://covid19.who.int
-
- CDC. Scientific Brief: SARS-CoV-2 Transmission [Internet]. Centers for Disease Control and Prevention. 2021 [cited 2022 Aug 21]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-co... - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
