Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: A large population-based study
- PMID: 36777324
- PMCID: PMC9903988
- DOI: 10.1016/j.lana.2022.100298
Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: A large population-based study
Abstract
Background: Population-based data on epidemiology of Inflammatory Bowel Diseases (IBD) in Brazil are scarce. This study aims to define temporal trends of incidence and prevalence rates of Crohn's disease (CD) and ulcerative colitis (UC) in Brazil.
Methods: All IBD patients from the public healthcare national system were included from January 2012 to December 2020. Average Annual Percent Change (AAPC) and 95% confidence intervals (CI) were calculated using log-linear regression for incidence and binomial regression for prevalence. Moran's I autocorrelation index was used to analyse clustering of cities by level of prevalence.
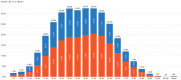
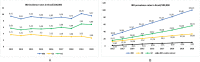
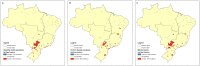
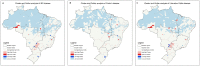
Findings: A total of 212,026 IBD patients were included. Incidence of IBD rose from 9.4 in 2012 to 9.6 per 100,000 in 2020 (AAPC=0.8%; 95% CI -0.37, 1.99); for UC, incidence increased from 5.7 to 6.9 per 100,000 (AAPC=3.0%; 95% CI 1.51, 4.58) and for CD incidence decreased from 3.7 to 2.7 per 100,000 (AAPC=-3.2%; 95% CI -4.45, -2.02). Prevalence of IBD increased from 30.0 in 2012 to 100.1 per 100,000 in 2020 (AAPC=14.8%; CI 14.78-14.95); for UC, from 15.7 to 56.5 per 100,000 (AAPC=16.0%; CI 15.94, 16.17); for CD from 12.6 to 33.7 per 100,000 (AAPC=12.1% CI 11.95, 12.02). A south-north gradient was observed in 2020 prevalence rates of IBD [I=0.40 (p<0.0001)], CD [I=0.22 (p<0.0001)] and UC [I=0.42 (p<0.0001)].
Interpretation: Incidence of CD is decreasing whereas of UC is increasing, leading to stabilization in the incidence of IBD from 2012 to 2020 in Brazil. Prevalence of IBD has been climbing with 0.1% of Brazilians living with IBD in 2020.
Funding: None.
Keywords: AAPC, Average Annual Percent Change; AC, Acre; AL, Alagoas; AM, Amazonas; AP, Amapá; BA, Bahia; Brazil; CAAE, Certificate of Presentation for Ethical Appreciation; CD, Crohn's disease; CE, Ceará; CI, confidence intervals; Crohn's disease; DATASUS, Department of Health Informatics/Ministry of Health; DF, Distrito federal; ES, Espírito Santo; Epidemiology; GO, Goiás; IBD, Inflammatory Bowel Diseases; IBDU, Inflammatory Bowel Diseases undetermined; IBGE, National Institute of Geographics and Statistics (Instituto Brasileiro de Geografia e Estatística); ICD-10, Classification of Diseases and Related Health Problems, Tenth Revision; Incidence; Inflammatory bowel disease; MA, Maranhão; MG, Minas Gerais; MS, Mato Grosso do Sul; MT, Mato Grosso; PA, Pará; PB, Paraíba; PE, Pernambuco; PI, Piauí; PR, Paraná; Prevalence; RN, Rio Grande do Norte; RO, Rondônia; RR, Roraima; RS, Rio Grande do Sul; SC, Santa Catarina; SE, Sergipe; SP, São Paulo; SUS, national public health system (Sistema Único de Saúde); TO, Tocantins; UC, ulcerative colitis; UNOESC, University of the West of Santa Catarina; Ulcerative colitis.
© 2022 The Authors.
Conflict of interest statement
Quaresma AB has received honoraria for speaking from AbbVie, Apsen and Janssen. Aderson OMCD has received honoraria for speaking from AbbVie, Janssen, Pfizer, and Takeda; he has been a consultant for Takeda, AbbVie, and Janssen. Coy CSR has no conflicts of interest. Magro DO has no conflicts of interest. Hino AAF has no conflicts of interest. Valverde DA is CEO of Techtrials a Healthcare Data Science Company. Panaccione R reports consulting from AbbVie, Abbott, Alimentiv (formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Schering-Plough, Shire, Sublimity Therapeutics, Theravance Biopharma, UCB, and Takeda Pharmaceuticals. He has received speaker's fees from AbbVie, Arena Pharmaceuticals, Celgene, Eli Lilly, Ferring, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Sandoz, Shire, and Takeda Pharmaceuticals. Coward SB has no conflicts of interest. Ng SC has received consulting and speaker fees from AbbVie, Ferring, Janssen, Menarini, and Takeda; served as a Scientific Advisory Board member for AbbVie, Ferring, and Takeda; and received research grants from AbbVie, Ferring, and Janssen. Kaplan GG has received honoraria for speaking or consultancy from AbbVie, Janssen, Pfizer, Amgen, and Takeda. He has received research support from Ferring, Janssen, AbbVie, GlaxoSmith Kline, Merck, and Shire. He has been a consultant for Gilead. He shares ownership of a patent: TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 Sept. 2018. Kotze PG has received consulting and speaker fees from AbbVie, Janssen, Pfizer, and Takeda. He also received scientific grants from Pfizer and Takeda.
Figures

References
-
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. - PubMed
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;23(10114):2769–2778. 390. - PubMed
-
- Goh KL, Xiao SD. Inflammatory bowel disease: a survey of the epidemiology in Asia. J Dig Dis. 2009;10(1):1–6. - PubMed
-
- Bernstein CN. Epidemiologic clues to inflammatory bowel disease. Curr Gastroenterol Rep. 2010;12(6):495–501. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

