Impact of KOH Activation on Rice Husk Derived Porous Activated Carbon for Carbon Capture at Flue Gas alike Temperatures with High CO2/N2 Selectivity
- PMID: 36777600
- PMCID: PMC9910064
- DOI: 10.1021/acsomega.2c06955
Impact of KOH Activation on Rice Husk Derived Porous Activated Carbon for Carbon Capture at Flue Gas alike Temperatures with High CO2/N2 Selectivity
Abstract
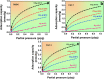
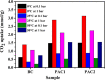
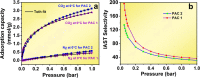
Metal-free porous activated carbon is an effective alternative to capture CO2 due to its high surface area and textural advantages. In this regard, the present research work explores a suitable method for producing activated porous carbon with a high specific surface area through a two-step reaction involving rice husk and KOH at 600 °C for 1 h to capture CO2. By varying the ratio of rice husk biomass to KOH, the texture and specific surface area of the activated porous carbon has been altered. A high surface area of ∼755 m2/g and a micropore volume of 0.243 cm3/g have been observed in the porous carbon produced with a KOH/biomass weight ratio of 3 (PAC2). Nitrogen contents in PAC1 and PAC2 were approximately 2.27 and 2.71 atom %, respectively. When compared with other materials, PAC2 has the highest CO2 adsorption capability, reaching up to 3.13 mmol/g at 0 °C and 1.55 mmol/g at 50 °C. The isosteric heat of adsorption confirms the presence of both physisorption and chemisorption. The materials turn out to be highly CO2/N2 selective, with the highest selectivity of 131, proving that the samples are potential materials for capturing CO2 from flue gases. These findings unequivocally show that porous activated carbon can be used to make CO2 adsorption efficient, inexpensive, and, more importantly, extremely effective.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Trends in Atmospheric Carbon Dioxide, Earth System Research Laboratory, National Oceanic & Atmospheric Administration. Https://Www.Noaa.Gov/News-Release/Carbon-Dioxide-Now-More-than-50-Higher... (accessed Sept 26, 2022).
-
- Yuan X.; Kumar N. M.; Brigljević B.; Li S.; Deng S.; Byun M.; Lee B.; Lin C. S. K.; Tsang D. C. W.; Lee K. B.; Chopra S. S.; Lim H.; Ok Y. S. Sustainability-Inspired Upcycling of Waste Polyethylene Terephthalate Plastic into Porous Carbon for CO2capture. Green Chem. 2022, 24, 1494–1504. 10.1039/d1gc03600a. - DOI
-
- Thengane S. K.; Bandyopadhyay S. Biochar Mines: Panacea to Climate Change and Energy Crisis?. Clean Technol. Environ. Policy 2020, 22, 5–10. 10.1007/s10098-019-01790-1. - DOI
-
- Gupta A.; Paul A. Carbon Capture and Sequestration Potential in India: A Comprehensive Review. Energy Procedia 2019, 160, 848–855. 10.1016/j.egypro.2019.02.148. - DOI
-
- Yu C. H.; Huang C. H.; Tan C. S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. 10.4209/aaqr.2012.05.0132. - DOI
LinkOut - more resources
Full Text Sources

